NNN = 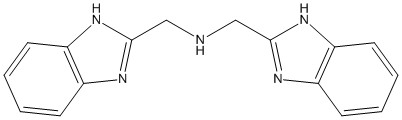
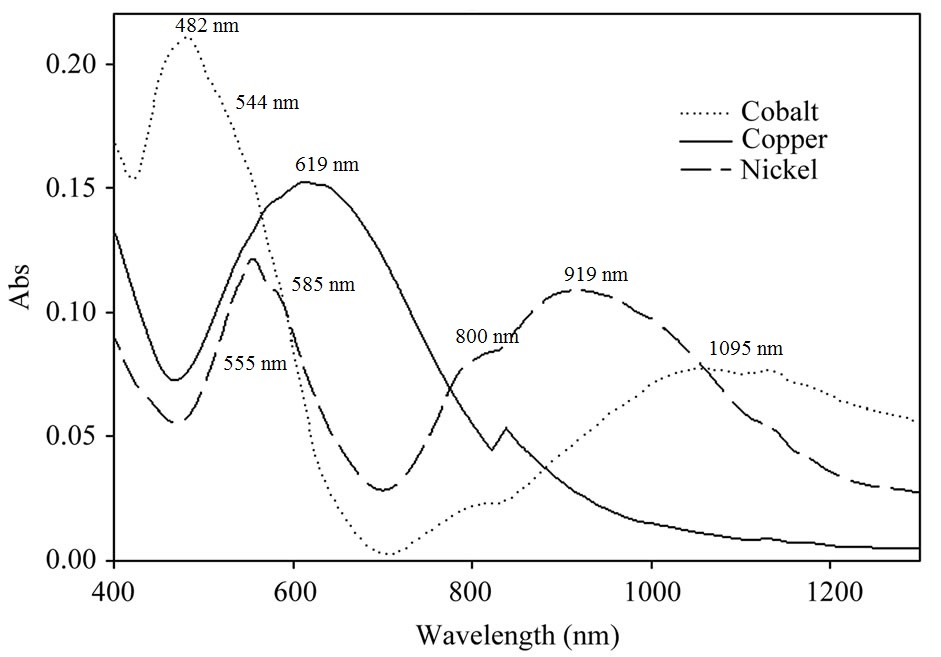
1. Below are the spectra of three metal complexes, [Co(NNN)2]2+, [Cu(NNN)2]2+, and [Ni(NNN)2]2+, where NNN is the tridentate ligand shown below.
NNN =
Find 10Dq, B, and the nephelauxetic parameter β for each complex (if possible) and assign the transitions for this complex assuming a pseudo-Oh geometry. Based on your values, which N atoms coordinate to the metal ions? Justify your conclusion.
The easiest of these is [Cu(NNN)2]2+, which is d9 and only a single transition is expected.
The peak maximum is at 619 nm or 16200 cm–1
For d9 the peak directly gives 10Dq = 16200 cm–1
There is not enough information available to determine B.
The transition is 2T2g ← 2Eg
[Ni(NNN)2]2+ is a d8 complex, where there is no high-spin/low-spin option.
There are 4 labeled peaks in the spectrum so there are several possible assignments
Guess 1
ν1 = 919 nm = 10900 cm–1
νspin-forbidden = 800 nm = 12500 cm–1
ν2 = 585 nm = 17100 cm–1
ν3 = 555 nm = 18000 cm–1
With this assignment, ν1 = 10Dq = 10900 cm–1
15B = ν3 + ν2 – 3ν1 = 18000 + 17100 – 3(10900) = 2400 cm–1 so B = 160 cm–1
β = Bcomplex/Bfree ion = 160/1080 = 0.148
B and β seem too low so this assignment is probably incorrect.
Guess 2
ν1 = 919 nm = 10900 cm–1
ν2 = 800 nm = 12500 cm–1
νspin-forbidden = 585 nm = 17100 cm–1
ν3 = 555 nm = 18000 cm–1
With this assignment, ν1 = 10Dq = 10900 cm–1
15B = ν3 + ν2 – 3ν1 = 18000 + 12500 – 3(10900) = –2200 cm–1 so B = –147 cm–1
B must be positive so this assignment is incorrect.
Guess 3
ν1 = 919 nm = 10900 cm–1
ν2 = 800 nm = 12500 cm–1
ν3 = 585 nm = 17100 cm–1
νspin-forbidden = 555 nm = 18000 cm–1
With this assignment, ν1 = 10Dq = 10900 cm–1
15B = ν3 + ν2 – 3ν1 = 17100 + 12500 – 3(10900) = –3100 cm–1 so B = –207 cm–1
B must be positive so this assignment is incorrect.
Guess 4: the closely spaced peaks arise from splitting because the complex is not Oh
ν1 arises from splitting of the 919 nm and 800 nm features so (averaging) ν1 = ½(10900 + 12500) = 11700 cm–1
ν2 arises from splitting of the 555 nm and 585 nm features so ν2 = ½(17100 + 18000) = 17550 cm–1
With this assignment, ν1 = 10Dq = 11700 cm–1
3B = (ν2 – ν1)(ν2 – ν1)/ (5ν2 – 9ν1) = (17550 – 2×11700)(17550 – 11700)/ (5×17550 – 9×11700) = 1950 cm–1 so B = 650 cm–1
β = Bcomplex/Bfree ion = 650/1080 = 0.602
These values seem most reasonable so this assignment is likely correct.
In Oh the assignments are ν1 3T2g ← 3A2g
and ν2 3T1g ← 3A2g
Assuming an approximate D3 symmetry the assignments would be: 919 nm 3E ← 3A2, 800 nm 3A1 ← 3A2, 585 nm 3A2 ← 3A2, and 555 nm 3E ← 3A2.
[Co(NNN)2]2+ is a d7 complex, so the complex may be high-spin or low-spin.
Guess 1 - High Spin. This would be consistent with the small values of 10Dq for the other two complexes
ν1 = 1095 nm = 9130 cm–1
ν2 = 544 nm = 18400 cm–1
ν3 = 482 nm = 20700 cm–1
10Dq = ν2 – ν1 = 18400 – 9130 = 9270 cm–1
15B = ν3 + ν2 – 3ν1 = 20700 + 18400 – 3(9130) = 11710 cm–1 so B = 781 cm–1
β = Bcomplex/Bfree ion = 781/1120 = 0.697
These seem reasonable
Guess 2 - Low Spin. This requires the use of the Tanabe-Sugano diagram.
ν1 = 1095 nm = 9130 cm–1
ν2 = 544 nm = 18400 cm–1
ν3 = 482 nm = 20700 cm–1
ν2/ν1 = 18400/9130 = 2.02
ν3/ν1 = 20700/9130 = 2.27
Looking at the Tanabe-Sugano diagram, at no point along the low spin side can the ν2/ν1 ratio be as high as 2. Thus, this assignment must be incorrect
Guess 3 - Low Spin. Assume that ν1 and ν2 overlap.
ν1, ν2 = 1095 nm = 9130 cm–1
ν3 = 482 nm = 20700 cm–1
ν3/ν1 = 20700/9130 = 2.27
Looking at the Tanabe-Sugano diagram, this ratio (= 40/18) could be attained at 10Dq/B = 22 (right at the high-spin/low-spin transition)
This gives B = 20700/40 = 520 cm–1 and B = 9130/18 = 510 cm–1, averaging to 515 cm–1
Then, 10Dq = 22B = 22(515) = 11300 cm–1
β = Bcomplex/Bfree ion = 515/1120 = 0.460
B and β are low but not completely unreasonable.
Based on the Irving-Williams series, 10Dq(Co2+) should be less than 10Dq(Ni2+). Both assignments fit this but the if the Co2+ complex is low spin, the values for 10Dq are essentially the same. Given the size of B and the Irving-Williams series, the assignment of the high-spin values is most likely.
Then, ν1, 1095 nm, 4T1g ← 4T1g
Then, ν2, 544 nm, 4T2g ← 4T1g
Then, ν3, 482 nm, 4A2g ← 4T1g
Coordination is probably between the central amine N atom and the imine N atoms in the 5-membered ring. The π system at the imine sites allows for some back donation, accounting for the low B value. The low 10Dq values must arise from steric repulsion in the ring system since both amine and imine donors would predict a strong field.
The spectra are taken from N. P. Magwa, E. Hosten, G. M. Watkins, Z. R. Tshentu, Int. J. Nonferr. Metals, 2012, 1, 49 – 58.