Homework 2
Due Tuesday, Feb. 19, 2019 at 3:15 p.m. EST
For the following, show the two lowest energy Lewis structures (identify the lower energy structure), estimate the bond angles, give the point group, and
give the predominant hybrid on the central atom.
a. azide ion, N3–
b. perrhenate ion, ReO4–
c. selenate ion, SeO42–
Answer
a. azide ion, N3–
Lewis Structures: 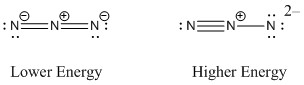
N-N-N bond angle: 180°
Point Group: D∞h
Hybrid: sp
b. perrhenate ion, ReO4–
Lewis Structures: 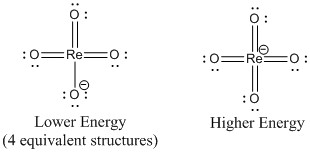
O-Re-O bond angle: 109.5°
Point Group: Td
Hybrid: sd3
c. selenate ion, SeO42–
Lewis Structures: 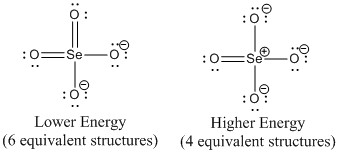
O-Se-O bond angle: 109.5°
Point Group: Td
Hybrid: sp3