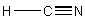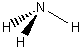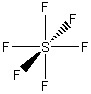Chemistry 401
Intermediate Inorganic Chemistry
University of Rhode Island
Practice Problems
Symmetry & Point Groups
1. Determine the symmetry elements and assign the point group of (a) NH2Cl, (b) CO32–, (c) SiF4, (d) HCN, (e) SiFClBrI, (f) BF4–.
Use VSEPR to find the structure and then assign the point group and identify the symmetry elements.
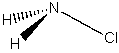
(a) NH2Cl
Point group = Cs
Symmetry elements: E, σ
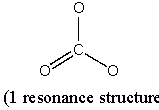
(b) CO32–
Point group = D3h
Symmetry elements: E, C3, C2, σh, σv, S3
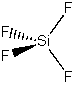
(c) SiF4
Point group = Td
Symmetry elements: E, C3, C2, σd, S4
(d) HCN
Point group = C∞v
Symmetry elements: E, C∞, σv
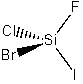
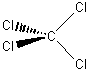
(e) SiFClBrI
Point group = C1
Symmetry elements: E
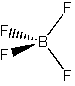
(f) BF4–
Point group = Td
Symmetry elements: E, C3, C2, σd, S4
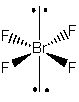
2. Determine the point group of SnF4, SeF4, and BrF4–.
Use VSEPR to find the structure and then assign the point group.
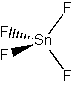
SnF4
Point group = Td
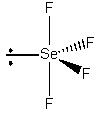
SeF4
Point group = C2v
BrF4–
Point group = D4h
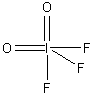
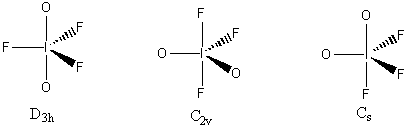
3. Consider a molecule IF3O2 (with I as the central atom). How many isomers are possible? Which is likely to have the lowest energy? Assign point group designations to each isomer.
The Lewis dot structure is:
The electron distribution is based on 5 sites, so a trigonal bipyramidal distribution is used. This leads to 3 different isomers (both O axial, both O equatorial, and 1 O axial and 1 O equatorial), as shown below with the appropriate point group.
The C2v structure is most likely the lowest energy because this minimizes the number of 90° repulsions between the I=O double bonds and the I-F single bonds.
4. Determine the point group for the following molecules: a) NH3; b) SF6; c) CCl4; d) H2C=CH2; e) H2C=CF2.
Find the structure of each molecule using VSEPR techniques.
a) NH3
C3v
b) SF6
Oh
c) CCl4
Td
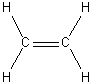
d) H2C=CH2
D2h
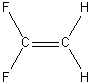
e) H2C=CF2
C2v
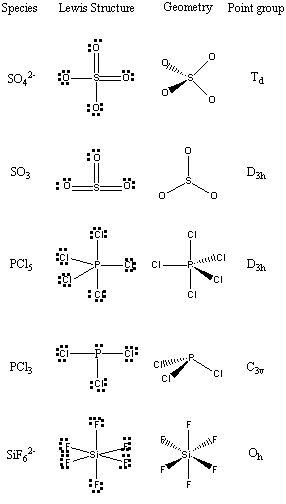
5. Find the point groups for the following species: SO42–, SO3, PCl5, PCl3, and SiF62–.
First, determine the structure of each species using VSEPR, then assign the point group.
6. Find the point group for the following molecules or ions: a) sulfur hexafluoride; b) fac–tribromotrichloroferrate(II) ion; c) tetraamminecopper(II) ion (ignore H atoms); d) decacarbonyldimanganese(0); e) chlorotris(triphenylphosphine)rhodium(I).
First, determine the structure using either the name or VSEPR, then the point group can be established.
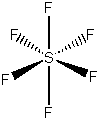
a) sulfur hexafluoride
VSEPR shows that the structure is
This belongs to the Oh point group.
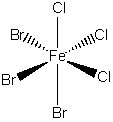
b) fac–tribromotrichloroferrate(II) ion
The name indicates that the structure is
This belongs to the C3v point group.
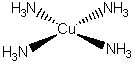
c) tetraamminecopper(II) ion (ignore H atoms)
The name indicates that the structure is
This belongs to the D4h point group.
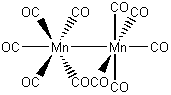
d) decacarbonyldimanganese(0)
The name indicates that the structure is
This belongs to the D4d point group.
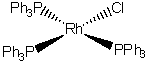
e) chlorotris(triphenylphosphine)rhodium(I)
The name indicates that the structure is
This belongs to the C2v point group.
7. Find the point group for the following species; a) mer–triamminetrichlorocobalt(III); b) tetracarbonylnickel(0); c) ferrocene.
a) mer–triamminetrichlorocobalt(III)
The structure is:
, which belongs to point group C2v
b) tetracarbonylnickel(0)
The structure is:
, which belongs to point group Td
c) ferrocene
The structure is:
, which belongs to point group D5d