Chemistry 401
Intermediate Inorganic Chemistry
University of Rhode Island
Practice Problems
Physical Techniques used in Inorganic Chemistry
1. How would you determine when the reaction of MgO and Al2O3 to produce MgAl2O4 was complete?
This reaction takes place in the solid state:
MgO(s) + Al2O3(s) → MgAl2O4(s)
X-ray diffraction is ideally suited to monitoring the reaction progress. As the reaction proceeds, the diffraction peaks for the reactants will disappear and the peaks for the product will appear. When all of the diffraction peaks associated with the reactants stop changing in intensity, the reaction is complete.
2. Calculate the wavelength associated with a neutron moving at 2.20 km s–1. Is this wavelength suitable for diffraction studies? (mn = 1.675 × 10–27 kg)
Using the de Broglie relationship:
λ = h/mv where:
h = Planck's constant = 6.626 × 10–34 J s
m = particle mass = 1.675 × 10–27 kg
v = particle velocity = 2.20 × 103 m s–1
λ = (6.626 × 10–34 J s)/(1.675 × 10–27 kg)(2.20 × 103 m s–1) = 1.80 × 10–10 m = 180 pm.
Since this is in the range of bond lengths, this is suitable for chemical diffraction studies.
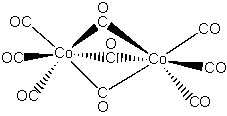
3. Explain why the 13C NMR spectrum of Co2(CO)9 shows only a single peak at room temperature.
The structure of Co2(CO)9 is shown below:
It appears that the terminal CO groups and the bridging CO groups should have different chemical shifts. Since only a single resonance peak is observed at room temperature, the CO groups must be rapidly exchanging positions on the NMR time scale.
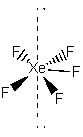
4. Explain the observation that the 19F NMR spectrum of XeF5– consists of a central peak symmetrically flanked by two peaks, each of which is roughly one-sixth of the intensity of the central peak.
The XeF5– ion is pentagonal planar, as shown below:
19F is 100 % abundant and has I = ½. Xe also has an I = ½ nucleus (129Xe) that is about 25 % abundant. All of the other Xe nuclei are I = 0. All of the 19F nuclei are chemically equivalent, so a single chemical shift is observed. For the 75 % of molecules that contain I = 0 Xe nuclei, this gives rise to a single peak in the 19F NMR spectrum. However, for the 25 % of ions that contain 129Xe, the 19F nuclei are split by the I = ½ 129Xe into two peaks. Thus, the relative intensities of the spectral peaks are 12.5 %, 75 %, and 12.5 %, or 6:1 between the central peak and the two wings.
5. How would you determine whether the compound Fe4[Fe(CN)6]3 contains discrete Fe(II) and Fe(III) sites?
This is an ideal situation for Mössbauer spectroscopy: Fe(II) and Fe(III) will have different isomer shifts and quadrupole splittings that would appear if there are discrete sites for each oxidation state. If only a single isomer shift were observed, then the oxidation states are averaged over all Fe sites.