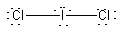Chemistry 401
Intermediate Inorganic Chemistry
University of Rhode Island
Fall 2019
Exam 1
1. Draw the Lewis structure, give the formal charge and oxidation number on each atom, predict the molecular structure, estimate all bond angles, and give the hybrid orbitals needed for the central atom(s)) for the following:
a. ICl2–
b. ClO2
c. IF5
a) ICl2–
Lewis structure:
Molecular structure: linear
Formal charges: I, –1; Cl (both), 0.
Oxidation numbers: I, +1; Cl (both), –1
Cl-I-Cl angle: 180°
Hybrid: sp3d
b) ClO2
Lewis structure:
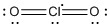
Molecular structure: bent planar
Formal charges: Cl, 0; O, 0.
Oxidation numbers: Cl: +4; O, –2.
O-Cl-O angles: ~108°
Hybrid: sp3
c) IF5
Lewis structure:
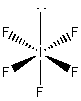
Molecular structure: square-based pyramid
Formal charges: I, 0; F (all), 0
Oxidation numbers: I, +5; F (all), –1
F-I-F angles: ~89° and ~179°
Hybrid: sp3d2
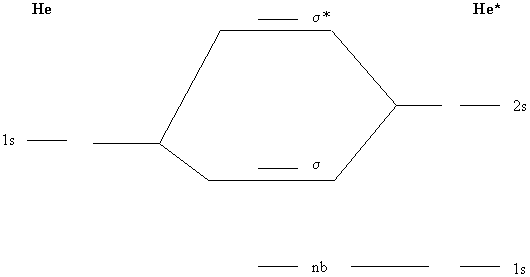
2. Construct a molecular orbital diagram for HeHe*, where He* is the excited state of He with electron configuration 1s12s1. What is the bond order of the molecule?
Use the 1s orbital on He and the 1s and 2s orbitals on He*. The MO diagram becomes:
The electron configuration is 1nb2σ2 so the bond order is ½(2 – 0) = 1.
3. Give the electron configuration and ground state term symbol for Ni, Ni2+, and Rh+.
Ni
Ni is [ar]4s23d8: L = 2 + 1 + 0 + –1 + –2 + 2 + 1 + 0 = 3 and S = ½ + ½ + ½ + ½ + ½ + –½ + –½ + –½ = 1 so the term symbol is 3F
Ni2+
Ni2+ is [Ar]3d8: L = 2 + 1 + 0 + –1 + –2 + 2 + 1 + 0 = 3 and S = ½ + ½ + ½ + ½ + ½ + –½ + –½ + –½ = 1 so the term symbol is 3F
Rh+
Rh+ is [Kr]4d8: L = 2 + 1 + 0 + –1 + –2 + 2 + 1 + 0 = 3 and S = ½ + ½ + ½ + ½ + ½ + –½ + –½ + –½ = 1 so the term symbol is 3F
4. Assuming that the atomic properties are valid in the solid state, predict the trend of the density for the first row transition metals, Sc to Zn. Briefly explain your reasoning.
As the elements go from left to right the atomic radius decreases (because Z* is increasing) and the atomic mass is increasing. Since density is proportional to mass/r3, from left to right the numerator is increasing and the denominator is decreasing. Both trends imply that the density should increase from Sc to Zn.
5. Ge and As are reported to have the same atomic radius, 122 pm. Provide an explanation for this anomaly.
Ge has an electron configuration of [Ar]4s23d104p2 and As has an electron configuration of [Ar]4s23d104p3. As has a larger Z*, which should reduce the atomic radius. Normally, the half-filled stabilization found in d and f elements does not apply to the p-block, if the atomic wavefunction for Ge has a contribution from the [Ar]4s13d104p3 configuration, the electron-electron repulsion is reduced, which allows a smaller radius.