Chemistry 401
Intermediate Inorganic Chemistry
University of Rhode Island
Fall 2018
Final Exam
All citations are to Inorg. Chem., 2018, 57
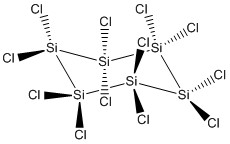
1. M. T. Frohlich, K. J. Anderson, A. Ugrinov, and P. Boudjouk (pages 14463 – 14666) report the synthesis of Si6Cl12, which is a strong enough Lewis acid to abstract halogens from carbon-halogen bonds. Draw the Lewis structure of this anion and predict all of the bond angles. Hint: the Si atoms form a ring. Give the point group.
Lewis structure (ignoring lone pairs on Cl atoms):
All of the Si centers are tetrahedral with no stereochemically active lone pairs so all of the bond angles are ~109°
Point group: D3d
2. A. Li, X. Zhang, Z. Xie, Z. Chang, Z. Zhou, and X.-H. Bu (pages 14476 – 14479) investigated catalysts for lithium/oxygen batteries. In these batteries the underlying redox reaction is thought to be Li + O2 → Li2O2, where the oxygen source is air. One of the problems is that carbon dioxide in air can cause Li2CO3 to form in an acid/base reaction, as well. Balance both reactions.
2 Li + O2 → Li2O2
Li2O2 + CO2 + H2O → Li2CO3 + H2O2
3. A. Naim, Y. Bouhadja, M. Cortijo, E. Duverger-Nédellec, H. D. Flack, E. Freysz, P. Guionneau, A. Iazzolino, A. O. Hamouda, P. Rosa, O. Stefanczyk, A. Valentin-Pérez, and M. Zeggar (pages 14501 – 14512) measured a variety of optical properties of [Fe(phen)3]2+. Give the systematic name, the point group, the LFSE in units of Dq and P, and the magnetic moment in units of Bohr-magnetons of this complex.
Name: trisphenthrolineiron(II) ion
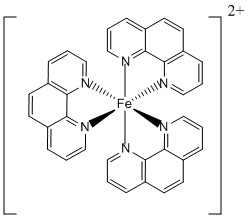
Point group: D3
LFSE: for d6 in a strong field LFSE = 24Dq – 2P
Magnetic moment: 0 unpaired spins so μ = 0 μB
4. D. Wen, H. Kato, M. Kobayashi, S. Yamamoto, M. Mitsuishi, K. Fujii, M. Yashima, and M. Kakihama (pages 14524 – 14531) synthesized as series of cerium compounds including Sr4.4Ce2.6EuZnO12. Determine the oxidation state and electron configuration of each ion in the oxide given that Eu is found as Eu3+.
Eu: oxidation state = +3; electron configuration = [Xe]4f6
O: oxidation state = –2; electron configuration = [Ne]
Sr: oxidation state = +2; electron configuration = [Kr]
Zn: oxidation state = +2; electron configuration = [Ar]3d10
Ce: oxidation state = +3.9, i.e. a mixture of +3 and +4; electron configurations = [Xe]4f1 (+3) and [Xe] (+4)
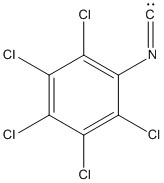
5. N. Bartalucci, L. Belpassi, F. Marchetti, G. Pampaloni, S. Zacchini, and G. Ciancaleoni (pages 14554 – 14563) calculated the molecular orbital properties of compounds related to pentachlorophenylisocyanideniobate(V). An isocyanide has the atom sequence of C-N-C6Cl5 and is a neutral ligand. Write the Lewis structure for the phenylisocyanide. One of the reasons that this class of complexes is interesting is that there is evidence of backbonding from the metal into the isocyanide ligand. Explain why this must be a controversial hypothesis.
Lewis structure:
Nb(V) has an electron configuration of d0 so there are no electrons to contribute for d to π* backbonding. The proposed answer is that the Cl atoms donate electron density into the Nb d orbitals, which then done into the isocyanide orbitals.
6. C. Jiang, M. Peng, A. M. Srivastava, L. Li, and M. G. Brik (pages 14705 – 14714) doped Mn4+ into CsNaGeF6. Write the Lewis structure, estimate the bond angles, and give the point group for the anion. Which cationic site would you expect the Mn4+ to occupy? Explain your reasoning. Determine the LFSE (in units of Dq and P) and the magnetic moment (in units of Bohr-magnetons) for Mn4+ at that site.
Lewis structure:
Bond angles: 90° and 180°
Point group: Oh
The Mn4+ will substitute for the Ge4+ sites because of the matching charges.
For [MnF6]2–, d3 in a weak field: LFSE = 12Dq and μ = [3(3+2)]½ μB = 3.87 μB
7. A. R. Jupp, T. C. Johnstone, and D. W. Stephan (pages 14764 – 14771) explored a way to measure Lewis acidity known as the Global Electrophilicity Index. Their calculations predict that the Lewis acid strength of the boron halides increases from fluoride to iodide, i.e. BI3 > BBr3 > BCl3 > BF3. Is this consistent with elementary periodic properties? Why or why not?
As the halides become larger (going down the Periodic Table) and more polarizable the boron halides will become softer acids. This means that BI3 will be the best Lewis acid for soft bases, using hard/soft acid/base principles. This matches the prediction. However, for hard bases the reverse should be true, i.e. BF3 should be the best Lewis acid for hard bases.
8. R. F. Brissos, P. Clavero, A. Gallen, A. Grabulosa, L. A. Barrios, A. B. Caballero, L. Korrodi-Gregório, R. Pérez-Tomás, G. Muller, V. Soto-Cerrato, and P. Gamez (pages 14786 – 14797) studied some ruthenium complexes that may be used as anticancer drugs. An example is η6-benzenebichlorotrimethylphosphineruthenium(II). Is this complex stable by the EAN rule? What is the point group?
EAN: Ru2+ is d6, η6-C6H6 contributes 6 e–, each Cl– contributes 2 e–, and :PMe3 contributes 2 e– so the total is 6 + 6 + 2(2) + 2 = 18 e–, which predicts stability
Structure:
which only has mirror symmetry so the point group is Cs
9. K. Matsumoto, Y. Haruki, S. Sawada, S. Yamada, T. Konno, and R. Hagiwara (pages 14882 – 14889) showed that the pentafluorosulfide anion, SF5–, had C4v symmetry when crystallized with glyme. Is this structure consistent with a VSEPR prediction? Why or why not?
The VSEPR predicted structure is
. The bond angles are slightly less than 90° but the 4-fold rotation axis and mirrors are maintained so the point group is C4v.
10. G. Jia and J. Du (pages 14961 – 14966) grew nanowires of CuInTe2, which act as semiconductors. What are the oxidation states and ground state term symbols for each atom in the compound? Elemental analysis indicated that the Cu was slightly substoichiometric. Does this suggest a p-type or n-type semiconductor? Explain your reasoning.
Te: oxidation state = –2 with electron configuration of [Xe] and term symbol 1S
In: oxidation state = +3 with electron configuration [Kr]4d10 and term symbol 1S
Cu: oxidation state = +1 with electron configuration [Ar]3d10 and term symbol 1S
Removing a small amount of Cu+ requires that some of the remaining Cu atoms be oxidized to Cu2+ to maintain charge neutrality. This introduces sites with extra positive charge so the semiconductor is p-type.