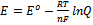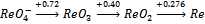Chemistry 401
Intermediate Inorganic Chemistry
University of Rhode Island
Fall 2017
Exam 2
1. The Kapustinskii equation, 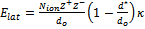 (Nion =
the number of ions in the formula unit and κ = 1.21×105 kJ·pm/mol), is an empirical method for
estimating lattice energies when the structure is not known. Compare the lattice energy found using the Kapustinskii
equation to that found from the Born-Mayer equation,
(Nion =
the number of ions in the formula unit and κ = 1.21×105 kJ·pm/mol), is an empirical method for
estimating lattice energies when the structure is not known. Compare the lattice energy found using the Kapustinskii
equation to that found from the Born-Mayer equation, 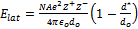 ,
where e = 1.602×10–19 C, 4πεo = 1.113×10–10
C2/J·mol) for CaF2. The experimental lattice energy is 2597 kJ/mol.
How do the two equations compare to the experimental value? Useful information: cation radius for
Ca2+ = 100 pm, anionic radius for F– = 133 pm, A = 2.519, and d* = 34.5 pm.
,
where e = 1.602×10–19 C, 4πεo = 1.113×10–10
C2/J·mol) for CaF2. The experimental lattice energy is 2597 kJ/mol.
How do the two equations compare to the experimental value? Useful information: cation radius for
Ca2+ = 100 pm, anionic radius for F– = 133 pm, A = 2.519, and d* = 34.5 pm.
2. Transition metal oxides often become semiconductors when exposed to oxygen, FeO being a typical example. Would you expect FeO after exposure to O2 to be an n-type or p-type semiconductor? Explain your reasoning.
3. Order the following species in order of increasing Brønsted-Lowry acid strength: K(H2O)6+, Fe(H2O)62+, Co(H2O)63+. Explain your reasoning.
4. In water, ammonia is a stronger Brønsted-Lowry base than pyridine. This question explores the Lewis base strength of these two compounds. Consider the reaction of each of these bases with the Lewis acids BF3 and CHCl3. Write the balanced equation for all four reactions and find ΔH° for each. Which is the stronger Lewis base, ammonia or pyridine? Explain your reasoning. –ΔH° = EAEB + CACB where:
SpeciesEC(giving units of kJ/mol)
BF320.23.31
CHCl36.180.32
NH32.787.08
C5H5N2.3913.10
5. The Latimer diagram for Re in acid is shown below. Write the balanced half reaction for the reduction of
ReO4– to the metal. What is the potential for the half-reaction under standard
conditions? What is the potential for the half-reaction at pH = 7 ([ReO4–] = 1.00 M,
T = 298 K, R = 8.314 J/mol·K, F = 96485 C/mol)? The Nernst equation is