Chemistry 401
Intermediate Inorganic Chemistry
University of Rhode Island
Fall 2013
Final Exam
All references are to volume 53 of Inorganic Chemistry (2013).
1. Ourane, Gaudin, Zouari, Couillard, and Bobet (pages 13289 – 13291) reported the structure of NdNiMg5, which is described as two interpenetrating networks of Mg and NdNi. In the Mg network the Mg-Mg distances are found to be 310.8 to 322.3 pm. Is this better described as metallic or covalent? Explain your reasoning. In the NdNi network two Nd-Ni distances were reported, 283.6 pm and 310.9 pm. Estimate the metallic radius and the covalent radius for Nd. Useful information: covalent radii, Mg 130 pm, Ni 120 pm; metallic radii, Mg 160 pm, Ni 125 pm.
The sum of the metallic radii for Mg is 320 pm while the sum of the covalent radii is 260 pm. The observed bond distances are near 320 pm so the Mg network is metallic.
The observed bond distances must be the sum of the radii for Nd and Ni. Metallic radii are longer than covalent radii so the longer bond distance must be a metallic bond and the shorter observed bond distance must be a covalent bond. Thus, the estimated metallic radius for Nd is 310.9 – 125 = 186 pm and the estimated covalent radius for Nd is 283.6 – 120 = 164 pm.
2. Cho, Joseph, and Rose (pages 13298 – 13300) used the ligand shown below to synthesize some cobalt complexes. Predict how this ligand would bind to Co2+.
There are two options for this ligand in binding to Co2+: acting as a tetradentate ligand, coordinating one Co2+ to all 4 N atoms, or acting as a bidentate ligand bridging two Co2+ ions. The bond angles of the CH2CH2 become quite strained in the tetradentate case so the structure is:
3. Mahesh, Rao, Thomas, Francis, and Koshy (pages 13204 – 13313) doped Eu3+ into some oxides to study the luminescent properties. Give the electron configuration and ground state term symbol for Eu3+.
Eu3+ is [Xe]6s05d04f6 so L = +3 + +2 + +1 + 0 + –1 + –2 = 3 and S = ½ + ½ + ½ + ½ + ½ + ½ = 3 so the term symbol is 7F
4. Pagola, Trowell, Havas, Reed, Chan, Van Dongen, and DeFotis (pages 13341 – 13350) determined the structure and magnetic properties for MnCl2·H2O and CoCl2·H2O. Each of these compounds is 6-coordinate (the chloride ions bridge between metals ions to fill the coordination sphere). Estimate the spin-only magnetic moment for each of these compounds. The observed magnetic moments at room temperature were 5.92 μB for MnCl2·H2O and 4.85 μB for CoCl2·H2O. Explain any differences between the observed and predicted magnetic moments.
MnCl2·H2O with weak ligands is high spin t2g3eg2 with 5 unpaired spins so μ = [5(5+2)]½ = 5.92 μB, which matches the experimental value.
CoCl2·H2O with weak ligands is high spin t2g5eg2 with 3 unpaired spins so μ = [3(3+2)]½ = 3.87 μB, which significantly less than the experimental value. The spin-only prediction breaks down because of orbital contributions or ferromagnetic contributions.
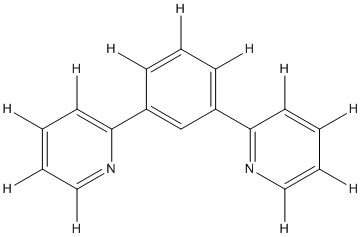
5. Dixon, Alary, Boggio-Pasqua, and Heully (pages 13369 – 13374) synthesized bis(η1-1,3-dipyridylbenzene)iron(II). Draw the structure, predict the stability, and give the point group of the compound. The ligand is shown below.
The structure of the complex is:
The point group is D2d
The complex is organometallic so the EAN rule should serve to predict stability: the Fe is d6, each N site contributes 2 electrons, and each C site contributes 2 electrons giving a total of 6 + 4(2) + 2(2) = 18, so stable.
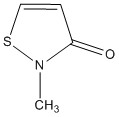
6. Kato, Unoura, Takayanagi, Ikeda, Fujihara, and Nagasawa (pages 13375 – 13383) showed that the ligand shown below is ambidentate, with coordination to either the S or O atoms. Show the preferred resonance structure for each mode of bonding. Why is coordination to the N atom not observed?
When bonding to the S atom the resonance structure is as shown:
When bonding to the O atom the resonance structure is:
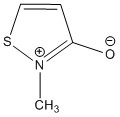
The N site is not readily available for binding to a metal because the resonance structure with a positive charge on the N atom is counterproductive for bonding to electropositive metals.
7. Pitchumony, Banevicius, Janzen, Zubieta, and Valliant (pages 13521 – 13528) studied the complex shown below. Give the name, the point group, and predict the stability of the ion.
Name: mer-2,2'-bipyridine-tris-carbonylpyridinerhenium(I)
Point group: Cs
Low valent so use the EAN rule: Re is d6, each CO contributes 2 electrons, and each N atom contributes 2 electrons, so the total = 6 + 3(2) + 3(2) = 18, so stable.
8. Jung, Ohkubo, Prokop-Prigge, Neu, Goldberg, and Fukuzumi (pages 13594 – 13604) studied redox reactions of Mn centers. Balance the reaction below, where L is a tetradentate cyclic ligand that does not change structure during the reaction. Use acidic conditions.
MnL + H2O2 + C6(CH3)6 → MnLO + C6(CH3)5CH2OH
There are two oxidations and one reduction:
oxidation: MnL + H2O → MnLO + 2 H+ + 2 e–
oxidation: C6(CH3)6 + H2O → C6(CH3)5CH2OH + 2 H+ + 2 e–
reduction: H2O2 + 2 H+ + 2 e– → 2 H2O
Multiply the reduction by 2 to remove the electrons to give the net reaction:
MnL + H2O2 + C6(CH3)6 → MnLO + C6(CH3)5CH2OH + 2 H2O
9. Seibel, Roudebush, Wu, Huang, Ali, Ji, and Cava (pages 13605 – 13611) characterized Na3Ni2BiO6. Find the oxidation state of each atom, given that the measured magnetic moment is 2.8 μB.
The measured magnetic moment suggests 2 unpaired spins on the Ni, which implies Ni2+. Then Na is +1, O is –2, leaving Bi to be +5.
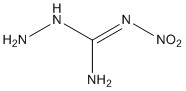
10. Fischer, Joas, Klapötke, and Stierstorfer (pages 13791 – 13802) synthesized 3-amino-1-nitroguanidine, shown below. Predict the most basic site and explain your reasoning.
The terminal NH2 on the NH is expected to be the most basic. The imine N is adjacent to the electron withdrawing nitro group, which reduces the electron density on the N atom. Likewise, the N atoms adjacent to the double bond will have some resonance structures with double bonds and positive charges on the N atoms, reducing their basicity.

.jpg)
.jpg)
2.jpg)
(py)(CO)3.jpg)