Chemistry 401
Intermediate Inorganic Chemistry
University of Rhode Island
Fall 2013
Exam 3
1. For the following compounds or ions, give the systematic name, the point group, the ligand field stabilization energy (in units of Dq), the predicted spin-only magnetic moment (in units of Bohr-Magnetons), and determine the predicted distortion if the complex is Jahn-Teller active:
a.
(Despite the drawing, the Fe-N-O and Fe-C-N bond angles are 180°.)
b.
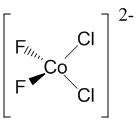
c.
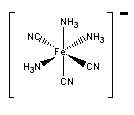
d.
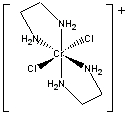
a.
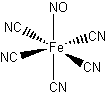
Name = pentacyanonitrosyliron(V)
Point Group = C4v
d3 in an Oh field so LFSE = 12Dq
μ = [3(3+2)]½ = 3.87 μB
Not Jahn-Teller active
b.

Name = bichlorobifluorocobaltate(II) ion
Point Group = C2v
d7 in a Td field so LFSE = 12Dqt
μ = [3(3+2)]½ = 3.87 μB
Not Jahn-Teller active
c.

Name = mer-triamminetricyanoferrate(II) ion
Point Group = C2v
d6 in a strong Oh field so LFSE = 24Dq – 2P
μ = 0 (no unpaired spins)
Not Jahn-Teller active
d.

Name = trans-bichlorobis(ethylenediamine)chromium(III) ion
Point Group = D2h (this assumes that the en rings are planar, which is not quite true)
d3 in an Oh field so LFSE = 12Dq
μ = [3(3+2)]½ = 3.87 μB
Not Jahn-Teller active
2. For the following compounds or ions, give the systematic name, the point group,and predict if the complex will be stable:
a.
b.
c.
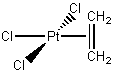
d.
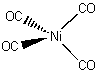
a.

Name = tetracarbonylnickel(0)
Point Group = Td
Ni(0) is d10, each CO contributes 2 electrons, so the total electron count is 10 + 4×2 = 18 therefore stable.
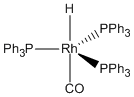
b.

Name = trans-carbonylhydridotris(triphenylphosphine)rhodium(I)
Point Group = C3v
Rh(I) is d8, the CO contributes 2 electrons, the hydride contributes 2 electrons, and each phosphorous contributes 2 electrons so the total electron count is 8 + 2 + 2 + 3×2 = 18 therefore stable.
c.
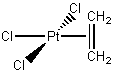
Name = trischloro-η2etheneplatinum(III)
Point Group = C2v
Pt(III) is d7, each Cl– contributes 2 electrons, and the ethene contributes 2 electrons so the total electron count is 7 + 3×2 + 2 = 15 therefore not stable.
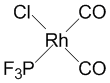
d.

Name = cis-bicarbonylchlorotrifluorophosphinerhodium(I)
Point Group = Cs
Rh(I) is d8, each CO contributes 2 electrons, the Cl– contributes 2 electrons, and the phosphorous contributes 2 electrons so the total electron count is 8 + 2×2 + 2 + 2 = 16 therefore probably stable.
3. For Co3+, the spin pairing energy is 21,000 cm–1 and Dq for NH3 (when bonded to Co3+) is 2,300 cm–1. Estimate the wavelength (in units of nm) for the lowest energy spin-allowed d-d transition in [Co(NH3)6]3+. Useful information: nm = 107/cm–1; ν = 10Dq for d6 high spin; and ν ~ 9Dq for d6 low spin.
10Dq = 23,000 cm–1 > 21,000 cm–1 = P, so the complex is low spin. Therefore ν = 107/(9×2100) = 483 nm.
