Chemistry 401
Intermediate Inorganic Chemistry
University of Rhode Island
Fall 2011
Exam 3
1. Give the systematic name for the following compounds: a) [Cu(NH3)4]2+; b) [Fe(CN)6]3–; c) [Cr(NH3)3Cl3] in the C3v point group.
a) [Cu(NH3)4]2+: tetraamminecopper(II) ion.
b) [Fe(CN)6]3–: hexacyanoferrate(III) ion
c) [Cr(NH3)3Cl3] in the C3v point group: fac-triamminetrichlorochromium(III)
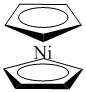
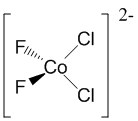
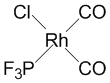
2. Give structures for the following compounds: a) bis-(η5-cyclopentadienyl)nickel(II); b) bichlorobifluorocobaltate(II) ion; c) cis-bicarbonylchloro(trifluorophosphine)rhodium(I).
a) bis-(η5-cyclopentadienyl)nickel(II):
b) bichlorobifluorocobaltate(II) ion:
c) cis-bicarbonylchloro(trifluorophosphine)rhodium(I):
3. For the following complexes, give the crystal field stabilization energy (in terms of Dq and P), the spin-only magnetic moment (in Bohr-Magnetons), and predict if the complex will be Jahn-Teller active. If the complex is Jahn-Teller active, indicate the likely nature of the distortion.
a. [CoF6]3–
b. [V(H2O)6]3+
c. [Mn(CN)6]3–
d. [Cr(H2O)3(CN)3]–
a. [CoF6]3–
Co3+ is d6 in a weak field so the stabilization energy is 4Dq. There are 4 unpaired electrons so μ = [4(4+2)]½ = 4.9 μB. d6 in a weak field is Jahn-Teller active and should display axial compression.
b. [V(H2O)6]3+
V3+ is d2 in a weak field so the stabilization energy is 8Dq. There are 2 unpaired electrons so μ = [2(2+2)]½ = 2.8 μB. d2 in a weak field is Jahn-Teller active and should display axial elongation.
c. [Mn(CN)6]3–
Mn3+ is d4 in a strong field so the stabilization energy is 16Dq – P. There are 2 unpaired electrons so μ = [2(2+2)]½ = 2.8 μB. d4 in a strong field is Jahn-Teller active and should display axial compression.
d. [Cr(H2O)3(CN)3]–
Cr2+ is d4 in a strong field (cyanide is a very strong ligand, water is near the changeover from weak to strong, so the stronger ligands dominate) so the stabilization energy is 16Dq – P. There are 2 unpaired electrons so μ = [2(2+2)]½ = 2.8 μB. d4 in a strong field is Jahn-Teller active and should display axial compression.
4. Indicate if the following complexes are likely to be stable. If not, suggest a simple reaction that could stabilize the structure.
a. [PtCl2(η2-CH2=CH2)]
b. [Mn(η5-cp)(CO)3] cp = cyclopentadienyl
c. [Cr(η6-C6H6)2]
d. [Fe(PF3)5]
These are all low valent complexes so the EAN rule can be used to predict stability.
a. [PtCl2(η2-CH2=CH2)]
Pt2+ is d8, contributing 8 electrons. Each chloride ion contributes 2 electrons and the ethylene contributes 2 electrons. The total electron count is 8 + 2(2) + 2 = 14, not expected to be stable. Reduction to the dianion could stabilize the complex.
b. [Mn(η5-cp)(CO)3] cp = cyclopentadienyl
Mn+ is d6, contributing 6 electrons. Each CO ligand contributes 2 electrons and the cp contributes 6 electrons. The total is 6 + 3(2) + 6 = 18, stable.
c. [Cr(η6-C6H6)2]
Cr0 is d6, contributing 6 electrons. Each benzene contributes 6 electrons. The total is 6 + 2(6) = 18, stable.
d. [Fe(PF3)5]
Fe0 is d8, contributing 8 electrons. Each PF3 contributes 2 electrons. The total is 8 + 5(2) = 18, stable.
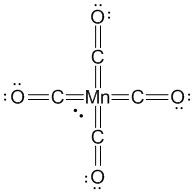
5. [Mn(CO)4]3– has a CO stretching frequency in the IR spectrum that is ketone like. Write a Lewis dot structure that accounts for this observation. Use the Lewis structure and the formal definition for finding oxidation states to determine the oxidation state of the Mn in this ion.
The ketone like CO stretch indicates that the carbonyl should be considered as a double bond as a result of unusually high back-bonding. A Lewis structure that accounts for this is:
The formal way to find the oxidation state from a Lewis structure is to assign all electrons in a bond to the more electronegative element. In this case, the O atoms are all assigned 8 electrons, the C atoms are all assigned 4 electrons, and the Mn atom is assigned 2 electrons, making the oxidation state +5. In comparison, assigning CO as a neutral ligand would imply an oxidation state of –3 for the Mn.