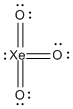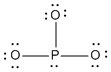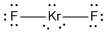Chemistry 401
Intermediate Inorganic Chemistry
University of Rhode Island
Fall 2011
Exam 1
1. Find the electron configuration and ground state term symbol for a) Na–; b) Mg2+; c) Co3+; d) La+; e) Cu2+. Use the rare gas notation for closed shells.
a) Na–
[Ne]3s2
This is a closed shell ion so L = 0 and S = 0 giving 1S
b) Mg2+
[Ne]
This is a closed shell atom so L = 0 and S = 0 giving 1S
c) Co3+
[Ar]3d6
↑↓ ↑ ↑ ↑ ↑ so L = 2 and S = 2; giving 5D
+2 +1 0 –1 –2
d) La+
[Xe]4f2
↑ ↑ so L = 5 and S = 1 giving 3H
+3 +2 +1 0 –1 –2 –3
e) Cu2+
[Ar]3d9
↑↓ ↑↓ ↑↓ ↑↓ ↑ so L = 2 and S = ½ giving 2D
+2 +1 0 –1 –2
2. Write the Lewis dot structure showing the formal charges, predict the structure including an estimate of all bond angles, and indicate the likely hybrid orbital on the central atom for the following: a) XeO3; b) PO33–; c) KrF2.
a) XeO3
Lewis Structure:
Formal charges: Xe, 0; all three O, 0
Structure: pyramidal with bond angles O-Xe-O ~108°
Hybrid orbital on Xe: sp3
b) PO33–
Lewis Structure:
Formal charges: P, 0; O, –1
Structure: pyramidal with bond angles O-P-O, ~107°
Hybrid orbital on P: sp3
c) KrF2
Lewis Structure:
Formal charges: Kr, 0; F , 0
Structure: linear with bond angles: F-Kr-F, 180°
Hybrid orbital on Kr: dsp3
3. In the first row diatomic molecules, B2 and C2 are anomalous because B2 has 2 unpaired spins and C2 has no unpaired spins, 2 pi bonds and no sigma bonds. Explain these observations.
Use Figure 2.17 from the textbook as a guide (or any other MO diagram for the first row diatomics)
There is an interaction between the σ* orbital created from the 2s orbitals and the σ orbital created from the 2pz orbitals that causes the anitbonding orbital to decrease in energy and the bonding orbital to increase in energy, above the π orbitals. For B2 this leads to the highest energy 2 electrons filling degenerate π orbitals so that Hund's rule tells us that these electron are unpaired. C2 adds 2 more electrons into the π orbitals giving no unpaired electrons and a bond order of 2. However, examination of the bonding shows that the lowest energy σ and σ* orbitals (created from the 2s orbitals) cancel out when describing the bond order and that all of the bonding arises from π bonds.
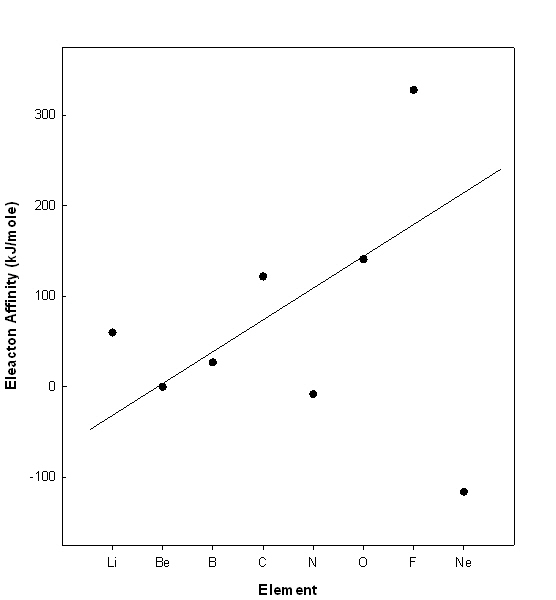
4. Plot the electron affinities for the first row atoms (Li to Ne). Explain the general trend and any deviations from the general trend.
The plot is given below.
The solid line shows the general trend, which is increasing from left to right on the Periodic Table, following Z*. The deviations are at Li, C, N, F, and Ne. Li, C, and F are above the trend line, which means that adding an electron to the element is more favorable than predicted. In each case this is because the product ion is either a filled or half-filled subshell (s2, s2p3, and s2p6, respectively). N and Ne are below the trend line because the element is a half-filled or filled subshell and adding an electron leads to a less favorable electron configuration (s2p4 for N and 2s22p63s1 for Ne).