Chemistry 401
Intermediate Inorganic Chemistry
University of Rhode Island
Fall 2010
Final Exam
All references are to Inorganic Chemistry, 49, 2010.
1. Kumar, Zhu, Walsh, and Prabhakar (pages 38 – 46) studied the use of Pd(H2O)42+ as a catalyst for peptide bond hydrolysis. Draw the structure, name, and find the point group of the complex.
Pd2+ in Pd(H2O)42+ is d8 and the complex is square planar (this is hard to predict, d8 complexes can also be tetrahedral).
Structure:
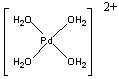
Name: tetraaquapalladium(II) ion
Point group: D4h
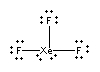
2. Grant, Wang, Dixon, and Christie (pages 261 – 270) determined the heats of formation of a number of xenon fluorides, including XeF3+ and XeF3–. Draw the Lewis dot structures for these two ions, estimate all of the bond angles, and give the point group of each.
XeF3+
Lewis structure:
The distribution is trigonal bipyramidal with the lone pairs occupying the equatorial positions:
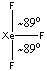
Point group: C2v
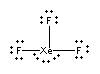
XeF3–
Lewis structure:
The distribution is octahedral with the lone pairs occupying the fac positions:
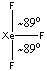
Point group: C2v
Interestingly, despite the charge and electron distribution difference, the two ions are predicted to have similar structures.
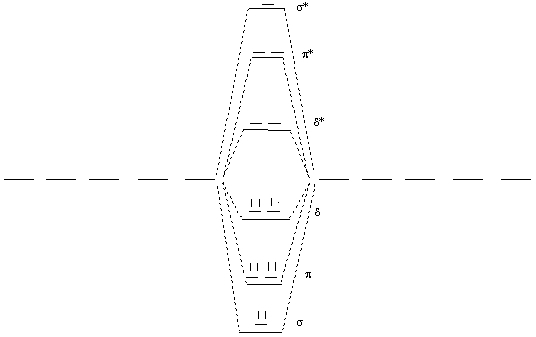
3. Cotton, Chiarella, Dalal, Murillo, Wang, and Young (pages 319 – 324) used EPR spectroscopy to find the type of orbital in which the unpaired electron in Os27+ resides. Draw a molecular orbital diagram for this ion, using only d valence orbitals, and predict the orbital occupation of the unpaired spin.
Os27+ has 9 valence electrons. The MO diagram is shown below:
The predicted electron configuration for the dimer is σ2π4δ3 so the unpaired electron is predicted to occupy a δ orbital.
4. Szajna-Fuller and Bakac (pages 781 – 785) investigated the reactions of rhodium hydrides with hydroxide ion and dioxygen. Consider a typical reaction, shown below:
LRhH+(aq) + OH–(aq) → LRh(aq) + H2O(l)
L represents ligands that occupy 5 coordination sites and have a net –1 charge. Identify the oxided and reduced species. Predict the product of the reaction of LRhH+ with dioxygen.
Rh is reduced from Rh(III) to Rh(I) and H– is oxidized to H+. O2 is an oxidizing agent, so can be expected to react with the hydride ion. In this case, the reaction is:
LRhH+(aq) + O
2(g) → LRhOOH+(aq) Other reactions could also be reasonably predicted.
5. Xu, Li, Xie, King, and Schaefer (pages 1046 – 1055) considered the use of BF as a ligand to substitute for CO. Fe(CO)4BF was their prototype compound. Write the Lewis dot structure for BF and predict which atom acts as the Lewis base end in the complex. Do you expect the iron complex to be stable by the EAN rule? Why or why not?
The Lewis dot structure for BF is:
. Since Z* is smaller for B, the B lone pair is expected to extend further into space, making the B end of the molecule the better Lewis base.
Fe(CO)4BF should have an electron count of Fe (d8) 8 electrons; CO, 2 electrons each for a total of 8 electrons; and BF 2 electrons, give a total of 8 + 8 + 2 = 18, predicted to be stable.
6. Derzsi, Dymkowski, and Grochala (pages 2735 – 2742) pondered the existence of a compound with stoichiometry AgSO4. They suggested three possibilities: Ag(II)SO4; Ag(I)2S2O8; and Ag(I)Ag(III)(SO4)2. Estimate the spin only magnetic moment (in units of Bohr-magnetons) for each representation of silver (II) sulfate. Could the spin moment be used to distinguish between the different possibilities? Why or why not?
Ag(I) is d10 with 0 unpaired spin, predicting μ = 0 μB.
Ag(II) is d9 with 1 unpaired spin, predicting μ = [1(1+2)]½ = 1.73 μB.
Ag(III) is d8 with 2 unpaired spins, predicting μ = [2(2+2)]½ = 2.83 μB.
Each of the three possible stoichiometries has a different magnetic moment, so determination of the magnetic properties can distinguish the possible oxidation states.
7. Lundberg, Persson, Eriksson, D'Angelo, and De Panfilis (pages 4420 – 4432) analyzed the ionic radii of Pm3+. Find the electron configuration and ground state term symbol for Pm3+. They found that the ionic radii of Pm3+ were: 1.146 Å for coordination number 9, 1.090 Å for coordination number 8, 1.054 Å for coordination number 7, and 0.971 Å for coordination number 6. Explain this trend.
Pm3+ has an electron configuration of [Xe]4f4.
L = 3 + 2 + 1 + 0 = 6 and S = ½ + ½ + ½ + ½ = 2 so the ground state term symbol is 5I.
The ionic radii increase with increasing coordination number, which is a reflection of steric crowding around the Pm3+ ion as more ligands are added. There is not enough room for larger numbers of ligands to get close to the Pm3+ ion so the ionic radius increases to make room for the increased coordination.
8. Singh and Xu (pages 6148 – 6152) studied the decomposition of hydrazine. Two possibilities are:
NH2NH2(aq) → N2(g) + H2(g)
NH2NH2(aq) → NH3(aq) + N2(g)
Balance each equation. Find the standard electrochemical potential under acidic conditions and under basic conditions. What is the preferred decomposition route under acid and base? Explain your decision for each condition.
The balanced reactions are:
NH2NH2(aq) → N2(g) + 2 H2(g)
3 NH2NH2(aq) → 4 NH3(aq) + N2(g)
The Latimer diagrams in the appendix can be used to answer the thermodynamic questions.
Under acidic conditions, the reactions are better written with the bases protonated:
NH2NH3+(aq) → N2(g) + 2 H2(g) + H+(aq)
The half-reactions are:
NH2NH3+(aq) → N2(g) + 5 H+(aq) + 4 e– E° = +0.23 V
2 H+(aq) + 2 e– → H2(g) E° = +0.00 V
The net potential is +0.23 V.
3 NH2NH3+(aq) + H+(aq) → 4 NH4+(aq) + N2(g)
The half-reactions are:
NH2NH3+(aq) + 3 H+(aq) + 2 e– → 2 NH4+(aq) E° = +1.275 V
NH2NH3+(aq) → N2(g) + 5 H+(aq) + 4 e– E° = +0.23 V
The net potential is +1.51 V.
In acid, the reaction significantly favors decomposition to ammonium ion and dinitrogen.
Under basic conditions:
NH2NH2(aq) → N2(g) + 2 H2(g)
The half-reactions are:
NH2NH2(aq) + 4 OH–(aq) → N2(g) + 4 H2O(l) + 4 e– E° = +1.16 V
2 H2O(l) + 2 e– → H2(g) + 2 OH–(aq) E° = –0.828 V
The net potential is +0.33 V.
3 NH2NH2(aq) → 4 NH3(aq) + N2(g)
The half-reactions are:
NH2NH2(aq) + 4 OH–(aq) → N2(g) + 4 H2O(l) + 4 e– E° = +1.16 V
NH2NH2(aq) + 2 H2O(l) + 2 e– → 2 NH3(aq) + 2 OH–(aq) E° = +0.1 V
The net potential is +1.3 V.
In base, the reaction also significantly favors decomposition to ammonia and dinitrogen.
9. Zeng, Gerken, Beckers, and Willner (pages 9694 – 9699) reported the synthesis and characterization of carbonyl diazide, CO(N3)2. Draw the Lewis structure showing nonzero formal charges and predict all of the bond angles for this compound.
There are 2 resonance structures of about the same energy:
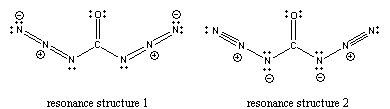
Predicted bond angles:
N-N-N: 180°, both structures
C-N-N: ~118°, resonance structure 1; ~107°, resonance structure 2
N-C-N: ~120°, resonance structure 1; ~122° (because of the charge repulsion on the N atoms), resonance structure 2
N-C-O: ~120°, resonance structure 1; ~119°, resonance structure 2
10. Gao (pages 10409 – 10414) investigated the size effects of small semiconductor clusters (these are also sometimes known as quantum dots). Predict electronic structure of the “bands” in a semiconductor that is not large enough to have completely delocalized metallic-type bonds. Hint: start with atoms and build the molecular orbitals.
As the basis orbitals overlap to become molecular orbitals, eventually the energies of the molecular orbitals become so close together that a quasicontinuum of states form, which we approximate as a continuous band of orbitals that electrons can occupy. To form the band requires a large number of orbitals, certainly a significant fraction of Avogadro's number of orbitals. When the number of orbitals is smaller, the band formation is incomplete, leaving energy gaps between states. This still allows formation of a valence band and conduction band, i.e., the conditions for a semiconductor, but the size of the band gap is controlled by the size of the particle. For smaller particles, the band gap is increased and is radius dependent.