Chemistry 401
Intermediate Inorganic Chemistry
University of Rhode Island
Fall 2010
Exam 3
1. For each of the following, draw the structure, determine the point group, find the LFSE (in terms of Dq and P), estimate the magnetic moment in terms of Bohr-Magnetons, and indicate if the complex will be Jahn-Teller active. If the complex is Jahn-Teller active, indicate the nature of the distortion.
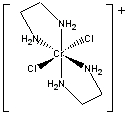
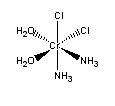
a. trans–bichloro–bis–ethylenediaminechromium(III) ion
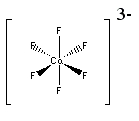
b. hexafluorocobaltate(III) ion
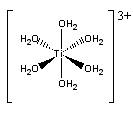
c. hexaaquatitanium(III) ion
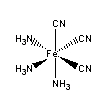
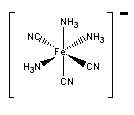
d. fac–triamminetricyanoiron(III)
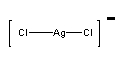
e. bichloroargentate(I) ion
a. trans–bichloro–bis–ethylenediaminechromium(III) ion
Structure:
Point group = D2h
The electron configuration is d3 in a weak field so LFSE = 12Dq
Unpaired spins = 3 so μ = [3(3+2)]½ = 3.87 μB
Not Jahn-Teller active
b. hexafluorocobaltate(III) ion
Structure:
Point group = Oh
The electron configuration is d6 in a weak field so LFSE = 4Dq
Unpaired spins = 4 so μ = [4(4+2)]½ = 4.90 μB
Jahn-Teller active – compression
c. hexaaquatitanium(III) ion
Structure:
Point group = Oh
The electron configuration is d1 in a weak field so LFSE = 4Dq
Unpaired spins = 1 so μ = [1(1+2)]½ = 1.73 μB
Jahn-Teller active – compression
d. fac–triamminetricyanoiron(III)
Structure:
Point group = C3v
The electron configuration is d5 in a strong field so LFSE = 20Dq – 2P
Unpaired spins = 1 so μ = [1(1+2)]½ = 1.73 μB
Jahn-Teller active – elongation
e. bichloroargentate(I) ion
Structure:
Point group = D∞h
The electron configuration is d10 in a weak field so LFSE = 0
Unpaired spins = 0 so μ = 0 μB
Not Jahn-Teller active
2. For each of the following, give the systematic name, determine the point group, find the LFSE (in terms of Dq and P), estimate the magnetic moment in terms of Bohr-Magnetons, and indicate if the complex will be Jahn-Teller active. If the complex is Jahn-Teller active, indicate the nature of the distortion.
a.
b.
c.
d.
e.
a.
Name: cis–biammine–cis–biaqua–cis–bichlorochromium(II)
Point group = C1
The electron configuration is d4 in a weak field so LFSE = 6Dq
Unpaired spins = 4 so μ = [4(4+2)]½ = 4.90 μB
Jahn-Teller active – either compression or elongation
b.
Name: mer–triamminetricyanoferrate(II) ion
Point group = C2v
The electron configuration is d6 in a strong field so LFSE = 24Dq – 2P
Unpaired spins = 0 so μ = 0 μB
Not Jahn-Teller active
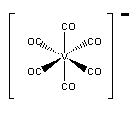
c.
Name: hexacarbonylvanadate(–I) ion
Point group = Oh
The electron configuration is d6 in a strong field so LFSE = 24Dq – 2P
Unpaired spins = 0 so μ = 0 μB
Not Jahn-Teller active
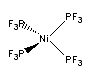
d.
Name: tetrakis–trifluorophosphinenickel(0)
Point group = Td
The electron configuration is d10 in a strong field so LFSE = 0
Unpaired spins = 0 so μ = 0 μB
Not Jahn-Teller active
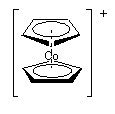
e.
Name: bis–cyclopentadienidecobalt(III) ion
Point group = D5d
The electron configuration is d6 in a strong field so LFSE = 24Dq – 2P
Unpaired spins = 0 so μ = 0 μB
Not Jahn-Teller active