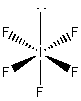Chemistry 401
Intermediate Inorganic Chemistry
University of Rhode Island
Fall 2010
Exam 1
1. Find the electron configuration and ground state term symbol for a) F; b) Ca; c) Ga3+; d) Bi; e) Pb2+. Use the rare gas notation for closed shells.
a) F
[He]2s22p5
↑↓ ↑↓ ↑ so L = 1 and S = ½ giving 2P
+1 0 –1
b) Ca
[Ar]4s2
This is a closed shell atom so L = 0 and S = 0 giving 1S
c) Ga3+
[Ar]3d10
This is a closed shell ion so L = 0 and S = 0 giving 1S
d) Bi
[Xe]4f144d106s26p3
↑ ↑ ↑ so L = 0 and S = 3/2 giving 4S
+1 0 –1
e) Pb2+
[Xe]4f145d106s2
This is a closed shell ion so L = 0 and S = 0 giving 1S
2. Write the Lewis dot structure showing the formal charges, predict the structure including an estimate of all bond angles, and indicate the likely hybrid orbital on the central atom for the following: a) SO3; b) SO32–; c) IF5.
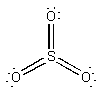
a) SO3
Lewis Structure:
Formal charges: S, 0; all three O, 0
Structure: trigonal planar with bond angles O-S-O 120°
Hybrid orbital on S: sp2
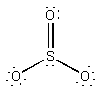
b) SO32–
Lewis Structure:
Formal charges: S, 0; two O, –1; one O, 0 (O average = –2/3)
Structure: pyramidal with bond angles O-S-O, ~107°
Hybrid orbital on S: sp3
c) IF5
Lewis Structure:
Formal charges: I, 0; F , 0
Structure: square based pyramid with bond angles: F-I-F, ~89°
Hybrid orbital on I: d2sp3
3. What are the expected changes in bond order and bond distance that accompany the following ionization process? Use MO theory to explain your answers.
(a) O2 → O2+ + e–
(b) N2 + e– → N2–
(c) NO → NO+ + e–
Use Figure 2.17 from the textbook as a guide (or any other MO diagram for the first row diatomics)
(a) O2 → O2+ + e–
O2 has a bond order of 2 and O2+ has a bond order of 2.5 (the electron has been removed from a π* orbital), so the bond order increases and the bond distance decreases.
(b) N2 + e– → N2–
N2 has a bond order of 3 and N2– has a bond order of 2.5 (the electron is put into a π* orbital) so the bond order decreases and the bond distance increases.
(c) NO → NO+ + e–
NO has a bond order of 2.5 and NO+ has a bond order of 3 (the electron is removed from a π* orbital) so the bond order increases and the bond distance decreases.
4. The first ionization potential for Cs is 3.9 eV and the first ionization potential for Au is 9.2 eV, quite different values. Yet the electron configuration for both elements is 6s1. Provide an explanation for the large differences in IP
The complete electron configurations are: Cs, [Xe]6s1 and Au, [Xe]4f145d106s1. The d and f electrons in Au provide poor shielding of the nuclear charge so Z* is much higher for Au than for Cs. Consequently, the 6s electron is more difficult to remove in Au.