Chemistry 401
Intermediate Inorganic Chemistry
University of Rhode Island
Fall 2009
Final Exam
All references are to volume 48 of Inorganic Chemistry (2009)
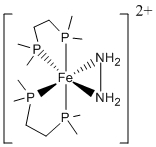
1. Field, Li, and Magill (pages 5 – 7) studied the reactivity of [Fe(dmpe)2(NH2NH2)]2+ (dmpe = 1,2-bis(dimethylphosphino)ethane), shown below. Find the point group for the complex. Reaction with a strong base deprotonates one hydrogen atom from each nitrogen of the hydrazine ligand. The product can be viewed either as an azene (HN=NH) ligand or as a dianionic hydrazido (NHNH2–) ligand. Consider each case: what is the oxidation state of the Fe and what is the LFSE? Could the two different representations be distinguished using uv-vis spectroscopy? If yes, how? If no, why not?
2. Haynes, Meijer, Lyons, and Adams (pages 28 – 35) examined the cis, mer isomer of [IrI3(CO)2Me]– (Me = methyl). Draw the structure and give the point group. Is the complex expected to be stable by the EAN rule? Why or why not?
3. Dethlefsen, Hedegård, Rimmer, Ford, and Døssing (pages 231 – 238) performed photochemical studies on the complex [Cr(NCCH3))5)(NS)]2+) (assume that the CrNS fragment is linear). Find the point group of the complex (ignore the hydrogen atoms). Photolysis causes the nitrogen monosulfide ligand to be released. Write the Lewis Dot structure for NS and find the formal charges and oxidation state on each atom. Do you think that the assumption that the CrNS fragment is linear is a good assumption? Why or why not?
4. Wu, Kroll, and Dias (pages 423 – 425) determined the crystal structure of chloro(η2-3-hexynegold(I). Draw the structure and find the point group. What drives the stability of this complex?
5. Zimmerman, Paul, Zhang, and Musgrave (pages 1069 – 1081) used ab initio calculations to study NH2BH2. Write the Lewis Dot structure for this compound using both the minimum formal charge rule and the octet rule, estimate all of the bond angles for each representation, and give the point group for each representation. NH2BH2 reacts readily at room temperature and can only be isolated at cryogenic conditions. Which dot structure is more consistent with this observation? Explain why.
6. Johns, Chmely, and Hanusa (pages 1380 – 1384) synthesized Ca[N(SiMe3)2]2 (Me = methyl), which is an ionic compound. Draw the Lewis Dot structure for the anion and give the point group. Which ion will form the lattice and which ion will fill the holes. Explain why.
7. Rogow, Swanson, Oliver, and Oliver (pages1533 – 1541) investigated the properties of (NH4)[Gd(CO3)2(H2O)]. Write the electron configuration for the gadolinium ion in this compound, give the term symbol for the electron configuration, and predict the spin-only magnetic moment. The observed magnetic moment is 3.02 μB. Explain any differences between the observed magnetic moment and the predicted magnetic moment.
8. Derossi, Brammer, Hunter, and Ward (pages 1666 – 1677) prepared [Ru(bpy)(CN)4]2– and studied its interactions with various cations. Write the structure, find the point group, determine the LFSE, predict the magnetic moment, and give the systematic name of the ion.
9. Zhang, Li, Xie, King, and Schaefer (pages 1974 – 1988) found that three isomers of Fe(CO)4(CS) lie close in energy (for linear Fe-C-X bonds, X = O, S). These isomers have point groups C2v, C3v, and C4v. Draw the structures and predict which is likely to be the most stable. Explain your reasoning.
10. Whaley, Rauchfuss, and Wilson (pages 4462 – 4469) suggested that the metal hydride [FeH(CN)2(CO)3]– could be used as a model compound for the active site in [NiFe]-hydrogenase, an enzyme used to reduce dihydrogen to hydrogen ions. fac-[FeH(CN)2(CO)3]– is prepared by adding a strong acid to D3h [Fe(CN)2(CO)3]2–. Write the acid/base reaction (showing structures of both complexes), find the point group for the product complex, and explain how adding a hydrogen ion to the reactant leads to a hydride product.