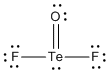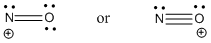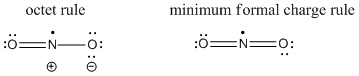Chemistry 401
Intermediate Inorganic Chemistry
University of Rhode Island
Fall 2009
Exam 1
1. Find the electron configuration and ground state term symbol for a) Co3+; b) Rh2+; c) Pt2+; d) Gd3+; e) Pb2+. Use the rare gas notation for closed shells.
a) Co3+
[Ar]3d6
↑↓ ↑ ↑ ↑ ↑ so L = 2 and S = 2 giving 5D
+2 +1 0 –1 –2
b) Rh+2
[Kr]4d7
↑↓ ↑↓ ↑ ↑ ↑ so L = 3 and S = 3/2 giving 4F
+2 +1 0 –1 –2
c) Pt2+
[Xe]4f145d8
↑↓ ↑↓ ↑↓ ↑ ↑ so L = 3 and S = 1 giving 3F
+2 +1 0 –1 –2
d) Gd3+
[Xe]4f7
↑ ↑ ↑ ↑ ↑ ↑ ↑ so L = 0 and S = 7/2 giving 8S
+3 +2 +1 0 –1 –2 –3
a) Pb2+
[Xe]4f145d106s2
Closed shell so L = 0 and S = 0 giving 1S
2. Write the Lewis dot structure showing the formal charges, predict the structure including an estimate of all bond angles, and indicate the likely hybrid orbital on the central atom for the following: a) TeOF2; b) BrF4–; c) ClO4–; d) PF3.
a) TeOF2
Lewis Structure:
Formal charges: Te, 0; O, 0; F, both 0
Structure: pyramidal with bond angles F-Te-F ~107° and F-Te-O ~108°
Hybrid orbital on Te: sp3
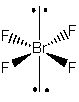
b) BrF4–
Lewis Structure:
Formal charges: Br, –1; F, all 0
Structure: square planar with bond angle F-Br-F, 90° and 180°
Hybrid orbital on Br: d2sp3
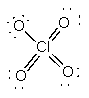
c) ClO4–
Lewis Structure:
Formal charges: Cl, 0; O (with double bonds), 0; O (with single bond), –1
Structure: tetrahedral with bond angles: O-Cl-O, ~109°
Hybrid orbital on Cl: sp3
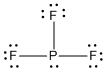
d) PF3
Lewis Structure:
Formal charges: P, 0; F, all 0
Structure: pyramidal with bond angles: F-P-F, ~107°
Hybrid orbital on P: sp3
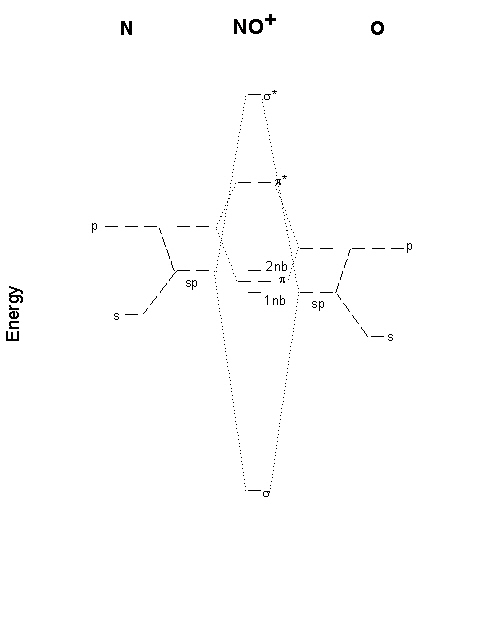
3. Draw a molecular orbital energy diagram for NO+. Find the bond order from your MO diagram and compare it to that predicted from the Lewis structure. Give the electron configuration.
The MO diagram with labels is shown below. Since NO+ is isoelectronic with CO, it is likely that sp hybridization is important. The electron configuration is σ21nb2π42nb2, which gives a bond order of 3.
Two possible Lewis structures are shown below. Using the octet rule gives a triple bond (bond order = 3), in agreement with the MO diagram but puts a positive charge on the more electronegative O atom. Using the Lewis structure that puts the positive charge on the N atom gives a double bond.
4. In a recent paper by R. F. See in the Journal of Chemical Education an analysis of the octet rule vs. the formal charge rule for Lewis structures was done. A theoretical description of the bond order using experimental bond lengths was compared to the two ways to draw Lewis structures. The table below gives a sampling of the results.
CompoundOctet Rule Bond Order Formal Charge Rule Bond OrderTheoretical Bond Order
NO21.521.53
PO21.521.88
O31.520.95
SO21.521.80
NF3110.82
PF3111.17
BCl31.3311.02
AlCls1.3311.03
Draw the two Lewis structures for NO2 and explain how the two different bond orders are found. Based on the data given, what conclusions can you come to about the best way to draw Lewis structures? Support your arguments using the data given.
The two Lewis structures are shown below:
The 'octet rule' structure (although there are only 7 electrons on the N atom, it is as close as one can get) has a nitrogen-oxygen double bond and a nitrogen-oxygen single bond. Since there are two resonance structures, each bond is an average of a single and double bond, i.e. a bond order of 1.5. The minimum formal charge structure has exclusively double bonds, so the bond order is 2. The problem with this structure is that there are 9 electrons on the N atom and there are no low energy vacant orbitals for all of these electrons to occupy.
In general, the theoretical bond order matches the octet rule better for first row atoms: NO2 has a theoretical bond order of 1.53, very close to 1.5 from the octet rule; for O3 the theoretical bond order does not match either Lewis prediction well, but is closer to the octet rule prediction. In contrast, when the central atom is from the second row, the minimum formal charge Lewis structure does a better job of predicting the bond order: for PO2, 1.88 theory vs. 2 minimum formal charge and SO2, 1.80 theory vs. 2 from the minimum formal charge. Even when the two Lewis structures predict the same bond order, NF3 and PF3, the second row atom has a higher theoretical bond order.
The exception to these conclusions is BCl3 where the octet rule does not match the theoretical prediction. This is because of the electron deficient nature of the B center and the reluctance of the Cl atom to make a formal double bond to the B atom. In this case, the formal charge rule is clearly better.