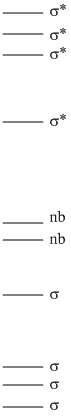Chemistry 401
Intermediate Inorganic Chemistry
University of Rhode Island
Fall 2008
Final Exam
All references are to volume 47 of Inorganic Chemistry (2008).
1. Vaqueiro (pages 20 – 22) prepared (C7H10N)(In9Se14) crystals (C7H10N = 3,5-dimethylpyridinium ion). Find the oxidation states and electron configurations for the In and Se in the anion.
The anion is In9Se14– and the likely oxidation states for In and Se are +3 and –2, respectively, based on the Periodic Table. To confirm this, 9×(+3) + 14×(–2) = –1, which matches the charge on the anion.
Electron configurations:
In3+: [Kr]4d10
Se2–: [Kr]
2. Tooyama, Braband, Spingler, Abram, and Alberto (pages 257 – 264) studied the chemistry of TcO4–. Write the Lewis dot structure for TcO4– and indicate the formal charge on each atom. Predict the reaction product of TcO4– with BF3·OEt2.
The Lewis dot structure is
with the formal charge for each atom labeled.
The reaction with boron trifluoride etherate is a Lewis acid/base substitution reaction:
TcO4– + BF3·OEt2 → [O3TcO–BF3]– + OEt2
3. Tregenna-Priggott (pages 448 – 453) investigated the Jahn-Teller distortion in Mn(III) complexes. His paper is aimed at explaining why tetragonal compression is a rare occurrence for Mn(III). Explain the factors that account for the idea that compression is rare for Mn(III).
Mn(III) is a d4 ion. If Mn(III) is low spin, then the Jahn-Teller distortion must be a compression, which implies that low spin Mn(III) is a rare situation. If the Mn(III) is high spin, which is common, then the simple rules predict either a compression or a distortion. However, occupation of the eg orbital is putting electron density into an antibonding orbital, which should weaken bond strengths and lead to longer bonds, i.e. elongation. Thus, any compression involving the occupation of eg orbitals must be rare.
4. Lau, et al, (pages 632 – 644) reacted cpCr(CO)3 (cp = η5-C5H5) with a variety of dithiadiazolyls. Name the complex and predict its stability.
cpCr(CO)3 = tricarbonyl-η5-cyclopentadienylchromium(I)
Cr(I) is d5, each CO contributes 2 electrons, and η5-C5H5 contributes 6 electrons, so the total electron count is 5 + 3(2) + 6 = 17; the complex is not predicted to be stable.
5. Krahl, Ahrens, Scholz, Heidemann, and Kemnitz (pages 663 – 670) solved the structure of (NH4)3GaF6 using NMR methods. Write the Lewis structure for each ion, predict all of the bond angles in each ion, and give the point group for each ion. The compound crystallizes in a cubic structure: which ion determines the lattice and which ion fills the holes. Explain your reasoning.
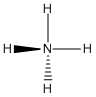
NH4+:
Lewis structure:
Bond angles: all H-N-H angles are ~109.5°
Point Group: Td
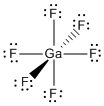
GaF63–:
Lewis structure:
Bond angles: all F-Ga-F angles are 90° or 180°
Point Group: Oh
GaF63– is a much larger ion, so will determine the lattice. NH4+ is smaller and will fill the holes.
6. Karaghiosoff, et al, (pages 1007 – 1019) examined the reactivity of the highly energetic methylated aminotetrazole salts. Balance the reaction: (C2H6N5)(NO3)(s) + O2(g) → CO2(g) + H2O(l) + N2(g). What is the energetic driving force for this reaction.
(C2H6N5)(NO3)(s) + 2 O2(g) → 2 CO2(g) + 3 H2O(l) + 3 N2(g)
Although this is an oxidation/reduction reaction, it can be readily balanced by inspection.
The driving force in this reaction is likely the formation of N2, which releases significant energy upon formation of the triple bond.
7. Zou, Xiang, Wei, Li, and Evans (pages 1361 – 1369) described a new synthetic route to ZnGa2O4, a wide band gap semiconductor. The band gap in this oxide is about 4.4 eV. What would you use to dope this material to reduce the band gap. Would this give a p-type or n-type semiconductor. Explain your reasoning.
Any number of dopants could be used: for example, Ge substituting for Ga would give a n-type semiconductor (Ge has more valence electrons than Ga) or excess Zn could substitute for Ga (Zn has fewer valence electrons than Ga) to give a p-type semiconductor.
8. Khater, Guillemin, Bajor, and Veszprémi (pages 1502 – 1511) investigated tellurols, RTeH. Write the Lewis dot structure for HTeCH3, predict the H-Te-C bond angle, and draw a qualitative MO diagram. The authors found two low energy peaks in the photoelectron spectrum of this compound at 8.46 and 11.00 eV. Assign the two observed peaks based on your MO diagram.
The Lewis structure is
. The H-Te-C angle may be reduced slightly from the ideal angle because of the lone pair repulsion, but Te is a large atom and the methyl group is also bulky, so a predicted angle is ~109°.
The MO diagram can be deduced from the Lewis structure: there must be 4 σ bonds (3 C-H and 1 Te-H), 4 σ antibonds, and 2 lone pairs. The C-H bonds will be lowest in energy (Z* on C) and the lone pairs are the highest energy.
The photoelectron spectrum peak at 8.46 eV arises from the nb (lone pair) electrons on the Te. The 11.00 eV is less clear: it could be from the second nb orbital or it could be from the Te-H σ orbital. The intensities of the peaks would help resolve the assignment: if the 8.46 eV peak is much more intense than the 11.00 eV peak, then the ionization from the two nb orbitals overlaps and the 11.00 eV peak must be from the Te-H bond. If the intensities of the two peaks is about the same, then the two ionizations arise from the two nb orbitals.
9. Welch, Holtrichter-Roessmann, and Stephan (pages 1904 – 1906) report the reaction of B(C6F5)3 with a number of phosphines. Estimate the enthalpy of reaction of trimethylphosphine with trimethylboron. Would you expect B(C6F5)3 to be a stronger or weaker Lewis acid with respect to trimethylphosphine. Explain your reasoning.
(CH3)3P + B(CH3)3 → (CH3)3P–B(CH3)3
The enthalpy of reaction can be found from the Drago-Wayland equation to be: ΔH = –[(12.6)(17.2) + (3.48)(13.40)] = –263.5 kJ/mol (quite a substantially exothermic reaction).
B(C6F5)3 has strongly electron withdrawing F atoms, which will reduce the electron density on the B atom, which should increase the Lewis acidity. However, the pentafluorophenyl group is also bulky, which would provide a steric repulsion in reaction with trimethylphosphine and reduce the Lewis acidity. Compared to trimethylboron, the pentafluorophenyl group is only slightly larger that the methyl group so the decrease in electron density probably dominates and B(C6F5)3 is a slightly stronger Lewis acid.
10. Mukherjee, Lloret, and Mukherjee (pages 4471 – 4480) study a new ligand, [2,4-ditert-butyl-6-{[(2-pyridyl)ethyl](2-hydroxybenzyl)-aminomethyl}-phenol. They synthesized complexes with Co2+, Ni2+, and Cu2+ and found the following data:
Ionλmax (nm) ε (M–1cm–1)μeff (BM)
Co2+750 304.4
Ni2+680 702.9
Cu2+770 4001.3
Is the ligand a weak field or strong field? Explain your reasoning.
Ni2+ and Cu2+ are d8 and d9, respectively, which do not have optional spin states so can not be easily used to determine the field strength of the ligand. Co2+ is d7, which is t2g6eg1 with 1 unpaired spin in the low-spin state and t2g5eg2 with 3 unpaired spins in the high-spin state. 1 unpaired spin predicts a magnetic moment of μeff ~ [1(1+2)]½ = 1.7 BM while 3 unpaired spins predicts a magnetic moment of μeff ~ [3(3+2)]½ = 3.9 BM. The observed magnetic moment for Co2+, 4.4 BM, clearly indicates the high-spin configuration. This means that the ligand must be a weak field ligand.