Chemistry 401
Intermediate Inorganic Chemistry
University of Rhode Island
Fall 2007
Final Exam
All references are to Inorganic Chemistry, volume 46, 2007.
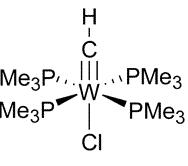
1. E. F. van der Eide, W. E. Piers, M. Parvez, and R. McDonald (pages 14 – 21) discussed the tungsten(IV) complex shown below. Would you expect this complex to be stable? Explain your reasoning.
Use the EAN rule: W(IV) is d2; each trimethylphosphine ligand contribute 2 e– for a total of 8, the chloride ion contributes 2 e–, and for the complex to be neutral the CH ligand must be –3 and the triple bond must contribute 6 e– for a total of 18 e–, which predicts that the complex should be stable.
2. S. Schneider>, M. Gerken, R. Haiges, T. Schroer, J. A. Boatz, D. A. Dixon, D. J. Grant, and K. O. Christe (pages 93 –102) investigated the reactivity of silyldichloramines. Write the Lewis dot structure for (CH3)3SiNCl2, predict the structure about each heavy atom, and estimate all of the bond angles.
The Lewis structure is:
.
The structure about each C atom and the Si atom is tetrahedral while the structure about the N atom is pyramidal. The estimated bond angles are H-C-H, 109°; C-Si-C, 109°; H-C-Si, 109°; C-Si-N, 109°; Si-N-Cl, 107°.
3. O. Gourdon, D. Gout, D. J. Williams, T. Proffen, S. Hobbs, and G. J. Miller (pages 251 – 260) did theoretical calculations on brass compositions. The ideal stoichiometry is Cu5Zn8. In this ideal situation, predict which atom will form the lattice and which holes the other atom will occupy. Explain your reasoning.
Zn has a larger atomic radius, 137 pm, compared to Cu, 128 pm, so the Zn atom should determine the lattice. While the Cu atom is smaller than the Zn atom, the size difference is fairly small so the Cu likely occupies the larger holes, i.e. the Td holes.
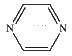
4. H. Bolvin (pages 417 – 427) studied the Creutz-Taube ion, [(NH3)5Ru-pyrazine-Ru(NH3)5]5+ (pyrazine is shown below). Estimate the LFSE for each Ru ion and determine the spin only magnetic moment.
In the limit of localized charges, the Ru have different oxidation states: Ru2+ and Ru3+. All of the ligands are strong field, so the electron configurations are t2g6 for the Ru2+ with LFSE = 24Dq – 2P and t2g5 for the Ru3+ with LFSE = 18Dq – 2P. Magnetic moments can not be separated onto the individual ions but the entire complex has 1 unpaired spin so μ = [1(1+2)]½ = 1.73 Bohr-magnetons.
5. W. Carrier, C. S. Jamieson, and R. I. Kaiser (pages 1332 – 1336) investigated trifluoromethyl sulfur pentafluoride, which is a greenhouse gas. Determine the point group of this molecule.
The structure of trifluoromethyl sulfur pentafluoride is shown below:
If the trifluoromethyl group is freely rotating, then the point group is C4v. If the triflouromethyl group is static and staggered from the pentafluorosulfur group, as shown in the graphic, then the point group is C1.
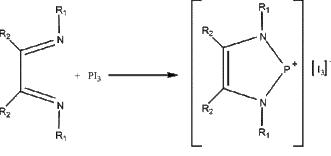
6. G. Reeske and A. H. Cowley (pages 1426 – 1430) studied the redox reaction shown below. Which species is oxidized and which is reduced? What about the product is unusual, hence interesting?
The I in PI3 is oxidized from the –1 oxidation number to the –1/3 oxidation number while the P in PI3 is reduced from the +3 oxidation number to the +2 oxidation number. The product has the P in a 2-coordinate, carbene-like state (a phosphenium ion), which is highly unusual and would be expected to invoke unique reactivity.
7. Z. Liu, Q.-S. Li, Y. Xie, R. B. King, and H. F. Schaefer III (pages 1803 – 1816) looked at vanadium carbonyl chemistry. V(CO)6 is not stable by the EAN rule (demonstrate this) while the predicted stable structure is V2(CO)12 (demonstrate this, too). Experimentally, the observed compound is V(CO)6. Propose an explanation why the divanadium compound is not observed.
V(0) in V(CO)6 is d5 and each CO donates 2 e– so V(CO)6 is a 17 e– complex, which is expected to be unstable. In V2(CO)12, at each V center, the V(0) is d5, each CO donates 2 e– and 1 e– is donated from a metal-metal bond: (CO)6V–V(CO)6, which gives a total of 18 e– on each V. The dimer probably does not form for steric reasons: each V is 7-coordinate in the dimer and there is not enough room around each V center to accomodate 7 nearest neighbors.
8. J. Köhler, S. Deng, C. Lee, and M.-H. Whangbo (pages 1957 – 1959) investigated the metal/semiconductor properties of NaTl. The Tl– ion forms a covalent structure similar to diamond or silicon. Explain why Tl– can behave like C or Si. Would you expect NaTl to be a metal or a semiconductor. Explain your reasoning.
Tl– has a s2p2 configuration just like C and Si, so similar structural behavior is not surprising.
Given the network structure of Tl–, one would expect that an electronic band structure similar to Si (or diamond) would develop. However, since the valence orbitals are 6s and 6p, which are quite close to each other in energy, the band gap in the Tl– electronic network is expected to be near or equal to zero. This predicts that NaTl will be a metal. (This is also the observation.)
9. K. Kadir and D. Noréus (pages 2220 – 2223) synthesized Mg2Na2NiH6, which contains the [NiH4]4– ion. Predict the structure of this ion and determine the LFSE.
The ligand in this case must be H– so that the Ni in [NiH4]4– must be Ni(0) with a d10 electron configuration. This implies a main group like structure, i.e. tetrahedral, and LFSE = 0.
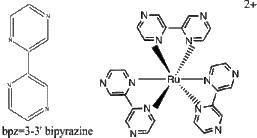
10. F. Alary, J.-L. Heully, L. Bijeire, and P. Vicendo (pages 3154 – 3165) considered the complex shown below. Name the ion and find the point group.
Name = tris(3,3'-bipyrazine)ruthenium(II) ion. Point group = D3.