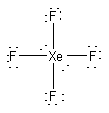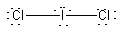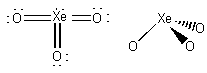Chemistry 401
Intermediate Inorganic Chemistry
University of Rhode Island
Fall 2007
Exam 1
1. The Pauling electronegativity generally decreases going down the Periodic Table. Ga and Ge are exceptions to this conclusion. Suggest an explanation for the anomaly for Ga and Ge.
Ga and Ge are the first elements after the first filled d subshell. The poor shielding of the d electrons is probably the reason, although there is no well resolved answer to this question. Pauling electronegativities are found from thermochemical bond energy data and Ga and Ge may have somewhat larger bond strengths (hence higher electronegativities) because, being at the metal/nonmetal border, they have both ionic and covalent character in their bonds. This trend for Ga and Ge does not appear in Allred-Rochow electronegativities that are based on Z* and covalent radii.
2. Find the electron configuration and ground state term symbol for a) Cr3+; b) Nb+; c) Cl–; d) Cu3+.
a) Cr3+
[Ar]3d3
↑ ↑ ↑ so L = 3 and S = 3/2 giving 4F
+2 +1 0 –1 –2
b) Nb+
[Kr]4d4
↑ ↑ ↑ ↑ so L = 2 and S = 2 giving 5D
+2 +1 0 –1 –2
c) Cl–
[Ar]
Closed shell so L = 0 and S = 0 giving 1S
d) Cu3+
[Ar]3d8
↑↓ ↑↓ ↑↓ ↑ ↑ so L = 3 and S = 1 giving 3F
+2 +1 0 –1 –2
3. Label (1s, 2s, etc.) the following atomic orbitals based on their nodal properties: a) 0 radial nodes, 3 planar nodes; b) 2 radial nodes, 2 planar nodes; c) 4 radial nodes, 1 planar node.
a) 0 radial nodes, 3 planar nodes: 4f
b) 2 radial nodes, 2 planar nodes: 5d
c) 4 radial nodes, 1 planar node: 6p
4. Write the Lewis dot structure showing the formal charges, predict the structure including an estimate of all bond angles, and indicate the likely hybrid orbital on the central atom for the following: a) XeF4; b) ICl2–; c) SO32–; d) XeO3.
a) XeF4
Lewis Structure:
Formal charges: Xe, 0; F, all 0
Structure: Square planar with bond angles F-Xe-F 90° and 180°
Hybrid orbital on Xe: d2sp3
b) ICl2–
Lewis Structure:
Formal charges: I, –1; Cl, both 0
Structure: Linear with bond angle Cl-I-Cl, 180°
Hybrid orbital on I: dsp3
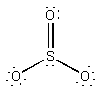
c) SO32–
Lewis Structure:
Formal charges: S, 0; O (with double bond), 0; O (with single bonds), –1
Structure: Pyramidal with bond angles: O-S-O, ~107°
Hybrid orbital on S: sp3
d) XeO3
Lewis Structure:
Formal charges: Xe, 0; O, 0
Structure: Pyramidal with bond angles: O-Xe-O, ~109°
Hybrid orbital on Xe: sp3
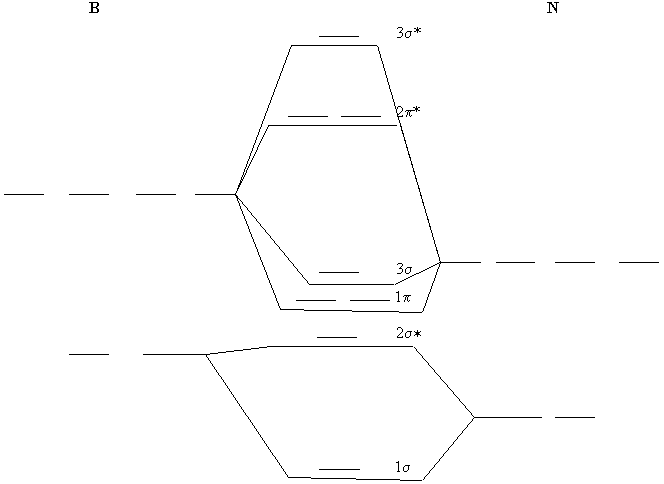
5. Construct the molecular orbital energy diagram for BN. Using your MO diagram, determine the bond order. How does this compare to the bond order expected from a Lewis dot structure? Explain any difference.
One possible MO diagram is shown below, using no hybridization. Since we have no data about BN, further refinement is challenging.
The bond order is 2, inconsistent with the triple bond found from the Lewis structure, B≡N: . This means that some sp hybridization must exist.