Chemistry 401
Intermediate Inorganic Chemistry
University of Rhode Island
Fall 2006
Exam 1
1. The first electron affinity for oxygen is +141 kJ/mol while the second electron affinity is –780 kJ/mol. Briefly account for the difference in magnitude and sign for these two values.
The attachment of the first electron is favorable, as is typical for most atoms. The second electron affinity is unfavorable (the negative sign) and large: this arises because the second electron is being added to an anion. The electron-electron repulsion makes it difficult to form the dianion.
2. Find the electron configuration and ground state term symbol for a) Ni3+; b) Rh3+; c) Se2–; d) W3+; e) Ag+.
a) Ni3+
[Ar]3d7
↑↓ ↑↓ ↑ ↑ ↑ so L = 3 and S = 3/2 giving 4F
+2 +1 0 –1 –2
b) Rh3+
[Kr]4d6
↑↓ ↑ ↑ ↑ ↑ so L = 2 and S = 2 giving 5D
+2 +1 0 –1 –2
c) Se2–
[Kr]
Closed shell so L = 0 and S = 0 giving 1S
d) W3+
[Xe]4f145d3
↑ ↑ ↑ so L = 3 and S = 3/2 giving 4F
+2 +1 0 –1 –2
e) Ag+
[Kr]4d10
Closed shell so L = 0 and S = 0 giving 1S
3. Polarizability is the ability of an electron cloud in an atom or molecule to be distorted by an electric field. How should polarizability relate to Z*? Briefly explain.
As Z* increases the electron cloud is held more closely to the nucleus (greater Coulomb attraction), is of smaller volume, and is more difficult to distort. Thus, polarizability should decrease as Z* increases.
4. Write the Lewis dot structure showing the formal charges, predict the structure including an estimate of all bond angles, and indicate the likely hybrid orbital on the central atom for the following: a) H2CO; b) AsF6–; c) ClO4–; d) SO3.
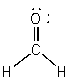
a) H2CO
Lewis Structure:
Formal charges: C, 0; H, 0; O, 0
Structure: Trigonal planar with bond angles H-C-H, ~118°; H-C-O, ~121°
Hybrid orbital on C: sp2
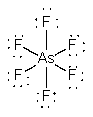
b) AsF6–
Lewis Structure:
Formal charges: As, –1; F, 0
Structure: Octahedral with bond angles F-As-F, 90°
Hybrid orbital on As: d2sp3
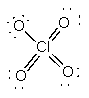
c) ClO4–
Lewis Structure:
Formal charges: Cl, 0; O (with double bonds), 0; O (with single bond), –1
Structure: Tetrahedral with bond angles: O-Cl-O, 109°
Hybrid orbital on Cl: sp3
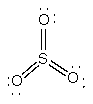
d) SO3
Lewis Structure:
Formal charges: S, 0; O, 0
Structure: trigonal planar with bond angles: O-S-O, 120°
Hybrid orbital on S: sp2
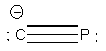
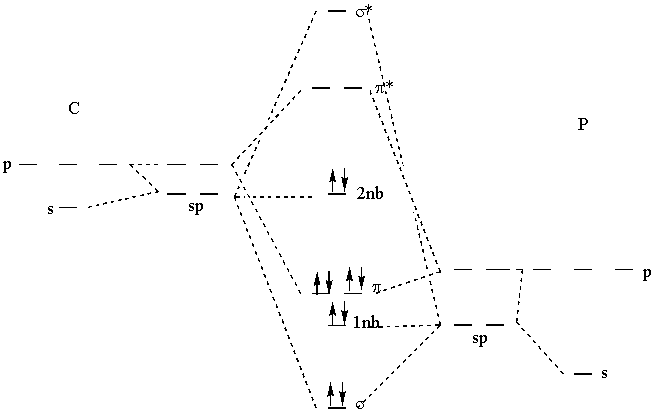
5. About 2 months ago the first report of the cyaphide anion was made. Cyaphide is the phosphorous analog to the cyanide ion, with phosphorous replacing nitrogen. a) Draw the Lewis dot structure for the cyaphide ion and give the formal charges on each atom. Are the formal charges consistent with electronegativity? b) Draw a molecular orbital energy diagram for the cyaphide ion. Give the bond order. Is the bond order consistent with the Lewis structure?
a) Lewis structure:
The formal charges are –1 on C and 0 on P, which is consistent with electronegativities. The Pauling electronegativity for C is 2.55 and 2.19 for P. The larger electronegativity should support the negative charge, in agreement with the Lewis dot structure.
b) The MO diagram is shown below, using sp hybridization on both the C and the P. The bond order is 3, consistent with the triple bond found from the Lewis structure.