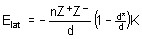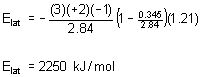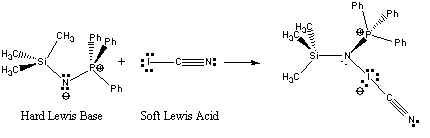Chemistry 401
Intermediate Inorganic Chemistry
University of Rhode Island
Fall 2001
Final Exam
All references are to articles from Inorganic Chemistry, 2001, 40.
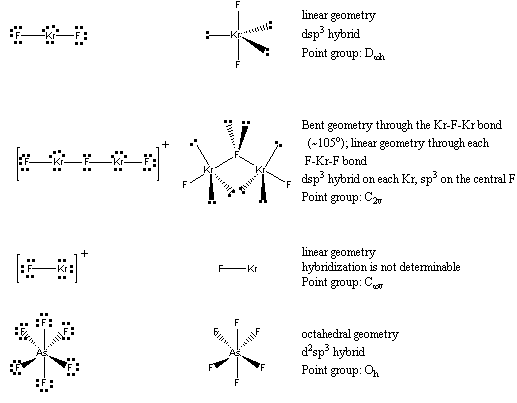
1. J. F. Lehmann, D. A. Dixon, and G. J. Schrobilgen (pages 3002-3017) prepared some compounds of Kr. Write the Lewis Dot structure, predict the molecular geometry, give the sigma hybridization, and indicate the point group for KrF2, Kr2F3+, and each ion in [KrF][AsF6].
2. R. Boca, M. Boca, L. Dihán, K. Falk, H. Fuess, W. Haase, R. Jarosciak, B. Papánková, F. Renz, M. Vrbová, and R. Werner (pages 3025-3033) studied the high-spin to low-spin transition in [Fe(bzimpy)2](ClO4)2·0.25H2O [bzimpz = 2,6-bis(benzimidazol-2-yl)pyridine)]. Find the LFSE for each spin state. Is either spin state likely to be Jahn-Teller active?
This is an Fe2+, d6 system. The LFSE for the high spin state is 4Dq and the LFSE for the low spin state is 24Dq – 2P. The high spin state would be Jahn-Teller active if the geometry around the Fe2+ were strictly octahedral, but since the two tridentate ligands lower the symmetry anyway, there may be no additional Jahn-Teller distortion.
3. B. Hoge, C. Thösen, and I. Pantenburg (pages 3084-3088) investigated the acid base reaction between HP(CF3)2 and Hg(CN)2 in the presence of dppe (Ph2PCH2CH2PPh2, Ph = phenyl). Write the acid base reaction (one of the products is [Hg{P(CF3)2}2dppe]). Why is HP(CF3)2 acidic? The role of the dppe is to stabilize the mercury complex. Explain how this may occur.
The acid base reaction is:
2 HP(CF3)2 + Hg(CN)2 + dppe → [Hg{P(CF3)2}2dppe] + 2 HCN
HP(CF3)2 is an acid because the trifluoromethyl groups strongly withdraw electron density from the H-P bond. Since the H-P bond is weakened, the ability to donate an H+ is enhanced, and the compound is an acid.
The Hg2+ in the product complex is d10 and the two [P(CF3)2]– ligands contribute 4 electrons to the metal center. The dppe is a chelating ligand and each P center in dppe contributes 2 electrons to the metal center. These additional 4 electrons give an 18 electron complex, which has increased stability.
4. X.-D. Jiao, P. D. Metelski, and J. H. Espenson (pages 3228-3233) looked at oxidation/reduction reactions between metal acetates and bromide ion. Balance the following reaction:
Co(CH3CO2)3(aq) + HBr(aq) → CoBr2(aq) + CH3CO2H(aq) + Br2(l)
Oxidation half-reaction:
2 HBr(aq) → Br2(l) + 2 H+(aq) + 2 e–
Reduction half-reaction:
Co(CH3CO2)3(aq) + 2 HBr(aq) + H+(aq) + e– → CoBr2(aq) + 3 CH3CO2H(aq)
Net reaction:
2 Co(CH3CO2)3(aq) + 6 HBr(aq) →2 CoBr2(aq) + 6 CH3CO2H(aq) + Br2(l)
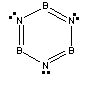
5. B. Kiran, A. K. Phukan, and E. D. Jemmis (pages 3615-3618) ask the question: is borazine aromatic? Borazine has the formula B3N3. Write the Lewis dot structure, give the point group, and give your opinion about the aromaticity in borazine. Defend your arguments.
The Lewis dot structure is:
The point group is D3h.
The molecule is planar, cyclic, and has 6 pi electrons in alternating double bonds, which is exactly the same description of the aromaticity in benzene. The counter argument to this is that the difference in electronegativity between B and N means that there must be an alternation of charge density (i.e., the B sites are relatively positive and the N sites are relatively negative).
6. G. V. Vajenine, G. Auffermann, Y. Prots, W. Schnelle, R. K. Kremer, A. Simon, and R. Kniep (pages 4866-4870) synthesized BaN2. Estimate the lattice energy of this compound using the Kapustinskii equation (page 57 in your text). You will need to estimate the ionic radius of the anion. To do this, assume a six-coordinate environment and estimate the ionic radius based on similar ions. Then estimate the heat of formation for BaN2 using the lattice energy you calculated. The sublimation energy for Ba(s) is 180.0 kJ/mol and all of the other data required is in your textbook. Based on the heat of formation, do you think the authors had an easy synthesis?
The Kapustinskii equation is:
n = number of ions in the formula unit = 3
Z+ = cation charge = +2
Z– = anion charge = –1
d = inter-ion distance = r+ + r–
r+ = 1.49 Å (Table 1.5, page 25 of Shriver & Atkins)
r– ~ 1.35 Å (Table 1.5, estimating the radius to be slightly larger than F–)
Then, d ~ 1.49 + 1.35 = 2.84 Å
d* = parameter to account for nuclear-nuclear repulsion = 0.345 Å (page 55 of Shriver & Atkins)
K = units conversion = 1.21 MJ·Å/mol
Inserting all of the values into the equation:
To find the heat of formation, ΔHf°, use the following Born-Haber cycle:
Ba(s) → Ba(g)S = sublimation energy = 180 kJ/mol (given in the problem)
Ba(g) → Ba2+(g)IP1 + IP2 = (5.21 + 10.00)×96.485 = 1468 kJ/mol (Table 1.6, page 27, Shriver & Atkins)
N2(g) → 2 N(g)D = bond dissociation energy = 946 kJ/mol (Table 3.5, page 72, Shriver & Atkins)
2 N(g) → 2 N–(g)–2×EA = –2×(–0.07)×96.485 = 14 kJ/mol (Table 1.7, page 29, Shriver & Atkins)
Ba2+(g) + 2 N–(g) → BaN2(s) –Elat = –2250 kJ/mol (from above)
Net reaction:
Ba(s) + N2(g) → BaN2(s) ΔHf°
Summing the energies in the thermodynamic cycle and solving gives:
ΔHf° = S + IP1 + IP2 + D – 2EA – Elat
ΔHf° = 180.0 + 1468 + 946 + 14 – 2250 = +358 kJ/mol
The rather large, positive heat of formation suggests that barium pernitride was difficult to synthesize.
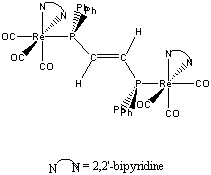
7. A. S. DelNegro, S. M. Woessner, B. P. Sullivan, D. M. Dattelbaum, and J. R. Schoonover (pages 5056-5057) investigated the photochemical reactivity of [(CO)3(bpy)Re(dppene)Re(bpy)(CO)3]2+ (dppene = trans-Ph2PCH=CHPPh2, Ph = phenyl, and has a systematic name of (E)-1,2-bis(diphenylphosphino)ethylene). Draw the structure of the complex, name the ion, and find the electron count around each Re atom. Do you expect the ion to be stable? Why or why not?
One possible structure (of several) is given below:
The systematic name is bis(2,2'-bipyridine)-μ-(E)-1,2-bis(diphenylphosphino)ethylene-fac-hexacarbonyldirhenium(I)
Each Re center is identical: Re+ is d6, each CO donates 2 electrons, each N on bpy donates 2 electrons, and the P from dppene donates 2 electrons for a total of 18 electron about each Re center. The complex should be stable, according to the 18 electron rule.
8. R. Kempe, E. Kessenich, and A. Schulz (pages 5182-5187) studied the Lewis acid-base reaction between (CH3)3SiNPPh3 (Ph = phenyl) and ICN. Identify the Lewis acid, the Lewis base, and write the structure of the product. Comment on the relative stability of the product.
The Lewis acid-base reaction is shown below:
Since the reaction is between a soft acid and a hard base, the adduct formed is not likely to be particularly stable.