Chemistry 401
Intermediate Inorganic Chemistry
University of Rhode Island
Fall 2001
Exam 2
1. Although I2 is an insulator under ambient conditions, with modest application of pressure it becomes a metal. Briefly explain why this happens in terms of an appropriate bonding theory.
The energy gap between the HOMO and LUMO in I2 is relatively small, as evidenced by the deep purple color. Upon application of pressure, the HOMO and LUMO orbitals overlap as the atoms are pushed closer together creating an energy band. Since the HOMO-LUMO gap is small, as the bands form this gap is reduced to zero allowing incomplete occupation of both bands. This is the requirement for a metal.
2. Diamond doped with nitrogen atoms is being considered for use in some semiconductor applications. Is this n-doped or p-doped? Explain your reasoning.
Nitrogen has extra valence electrons compared to carbon, so the diamond becomes n-doped. Another way to think about this is that each C atom in diamond has four single bonds to adjacent C atoms. When N is doped into the lattice it replaces some of the C atoms. But N only forms three single bonds, so one of the adjacent C atoms has an incomplete bond, i.e. a radical. The radical, a negatively charged electron, can move around the lattice from C atom to C atom rather freely.
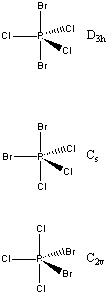
3. PBr2Cl3 has three possible isomers. Give the point group of each isomer.
The VESPR structures and point groups are:
4. Predict the products and estimate the enthalpy of reaction for:
F3B–S(CH3)2 + I2–P(CH3)3 →
Hint: use the data in Table 5.4, page 170 in your textbook to find the enthalpy for each complex and then find the reaction enthalpy.
First, use Hard-Soft Acid/Base theory to predict the products to be the result of a metathesis reaction (boron trifluoride is a hard acid, iodine is a soft acid, dimethyl sulfide is a soft base, and trimethylphosphine is a harder base):
F3B–S(CH3)2 + I2–P(CH3)3 → F3B–P(CH3)3 + I2–S(CH3)2
Using the hint, the enthalpy of complex formation can be found using the Drago-Wayland equation, –ΔH° = EAEB + CACB:
F3B + S(CH3)2 → F3B–S(CH3)2
ΔH° = –[(20.2)(0.70) + (3.31)(15.26)] = –64.65 kJ/mol
I2 + P(CH3)3 → I2–P(CH3)3
ΔH° = –[(2.05)(17.2) + (2.05)(13.40)] = –62.73 kJ/mol
F3B + P(CH3)3 → F3B–P(CH3)3
ΔH° = –[(20.2)(17.2) + (3.31)(13.40)] = –391.79 kJ/mol
I2 + S(CH3)2 → I2–S(CH3)2
ΔH° = –[(2.05)(0.70) + (2.05)(15.26)] = –32.72 kJ/mol
Now, the reaction enthalpy is found by adding the complex formation enthalpies for the products and subtracting the sum of the complex formation enthalpies of the reactants:
ΔH° = [ΔH°(F3B–P(CH3)3) + ΔH° (I2–S(CH3)2)] – [ΔH°(F3B–S(CH3)2) + ΔH°(I2–P(CH3)3)] = [–391.79 + –32.72] – [–64.65 + –62.73] = –297.13 kJ/mol
The reaction is quite exothermic, as anticipated by the Hard-Soft Acid/Base prediction.
5. The following reaction can be balanced two ways. Balance the reaction both ways, give the standard potential for each balanced reaction, and estimate pKa1 and pKa2 for H2MoO4.
H2MoO4(s) + Mo(s) → Mo3+(aq) + MoO2(s)
Hint: use the data from Appendix 2, page 703.
There are two possible oxidation/reduction combinations: (a) Mo6+/Mo3+ (reduction) with Mo0/Mo4+ (oxidation) or (b) Mo6+/Mo4+ (reduction) with Mo0/Mo3+ (oxidation). Each combination gives a different balanced reaction and a different potential.
(a):
Reduction: H2MoO4(s) + 6 H+(aq) + 3 e– → Mo3+(aq) + 4 H2O(l) E°red = [2(0.646) + (–0.2)]/3 = +0.364 V
Oxidation: Mo(s) + 2 H2O(l) → MoO2(s) + 4 H+(aq) + 4 e– E°red = [3(–0.13) + (–0.2)]/4 =–0.15 V
Net reaction: 4 H2MoO4(s) + 3 Mo(s) + 12 H+(aq) → 4 Mo3+(aq) + 3 MoO2(s) + 10 H2O(l) E° = +0.354 – (–0.15) = + 0.614 V
(b):
Reduction: H2MoO4(s) + 2 H+(aq) + 2 e– → MoO2(s) + 2 H2O(l) E°red = +0.646 V
Oxidation: Mo(s) → Mo3+(aq) + 3 e– E°red = –0.13 V
Net reaction: 3 H2MoO4(s) + 2 Mo(s) + 6 H+(aq) → 2 Mo3+(aq) + 3 MoO2(s) + 6 H2O(l) E° = +0.646 – (–0.13) = + 0.776 V
Reaction (b) is more favored thermodyamically, but not by a large margin.
To estimate the pKa values for H2MoO4(s), use Pauling's rules:
H2MoO4(s) = (HO)2MoO2 so pKa1 ~ 8 – 5(2) = –2 and pKa2 ~ –2 + 5 = 3.