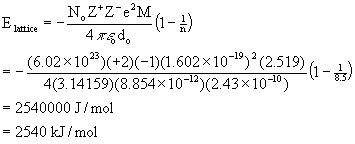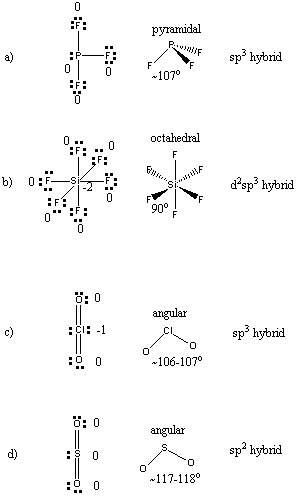Chemistry 401
Intermediate Inorganic Chemistry
University of Rhode Island
Fall 2001
Exam 1
1. Give the n, l, and all of the ml quantum numbers for the 3d, 4d, and 5d orbitals. Sketch the radial wave function as a function of distance from the nucleus on a single graph for all three orbitals.
3d: n = 3; l = 2; ml = +2, +1, 0, –1, –2.
4d: n = 4; l = 2; ml = +2, +1, 0, –1, –2.
5d: n = 5; l = 2; ml = +2, +1, 0, –1, –2.
Sketches of the radial wavefunctions:
2. Find the electron configuration and ground state term symbol for a) Fe3+; b) Mn; c) S2–; d) Cr3+; e) Cu+.
a) Fe3+: [Ar]3d5, 6S.
b) Mn: [Ar]4s23d5, 6S.
c) S2–: [Ar], 1S.
d) Cr3+: [Ar]3d3, 4F.
e) Cu+: [Ar]3d10, 1S.
3. Estimate the lattice energy for fluorite (CaF2). Ionic radii are found in Table 1.5 (pg. 25) and Madelung constants are found in Table 2.5 (pg. 55). The Born exponent has a value of 8.5.
In the fluorite structure the coodination number for the fluoride ion is 4 so the anionic radius (from Table 1.5) is 1.31 Å while the coordination number for the calcium ion is 8 so the cationic radius is 1.12 Å. This gives an estimated internuclear distance of 1.31 + 1.12 = 2.43 Å = 2.43×10–10 m. The Madelung constant given in Table 2.5 for the fluorite structure is 2.519 and the problem gives n = 8.5. This then leads to:
The experimental lattice energy is 2630 kJ/mol, in reasonably good agreement with the theory.
4. Write the Lewis dot structure showing the formal charges, predict the structure including an estimate of all bond angles, and indicate the likely hybrid orbital on the central atom for the following: a) PF3; b) SiF62–; c) ClO2–; d) SO2.
5. The MO diagram for cyanide ion calculated by a computer program is given below. Label each orbital as σ, σ*, π, π*, δ, or δ*. What is the bond order? Compare this to the bond order found from a Lewis structure.
The labeling of the orbitals is given below:
The bond order is 2. The Lewis structure for cyanide is :C:::N:, also giving a bond order of 3. The reason the two answers do not match is because the entire mo diagram (showing a lower energy bonding orbital) is not given.