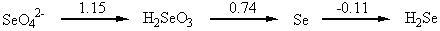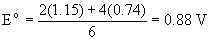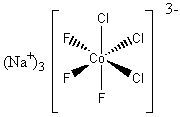Chemistry 401
Intermediate Inorganic Chemistry
University of Rhode Island
Fall 2000
Exam 3
1. The Drago-Wayland equation is designed for complex formation reactions, but can be used to determine the enthalpy of reaction for substitution and metathesis reactions by using the appropriate thermochemical cycle. Using the data from Table 5.4, estimate the enthalpy of the reaction ICl–NH3 + P(CH3)3 → ICl–P(CH3)3 + NH3. Hint: first find ΔHformation for each Lewis acid/base complex separately.
ICl EA = 10.4, CA = 1.70
NH3 EB = 2.78, CB = 7.08
P(CH3)3 EB = 17.2, CB = 13.40
giving units of kJ/mol
ICl + P(CH3)3 → ICl–P(CH3)3 [1]
–ΔH = (10.4)(17.2) + (1.70)(13.40) = 201.7 kJ
ICl + NH3 → ICl–NH3 [2]
–ΔH = (10.4)(2.78) + (1.70)(7.08) = 40.9 kJ
For
ICl–NH3 + P(CH3)3 → ICl–P(CH3)3 + NH3
ΔH = ΔH([1]) – ΔH([2]) = –201.7 – (–40.9) = –160.8 kJ
2. Use the Latimer diagram for Se in acidic solution (Appendix 2) to find the reduction potential for the reduction of SeO42– to Se. Write the balanced half-reaction.
The Latimer diagram for Se is
The desired reaction is
SeO42–(aq) + 8 H+(aq) + 6 e– → Se(s) + 4 H2O(l)
The standard reduction potential is found by the weighted average of the values given in the Latimer diagram:
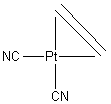
3. Give the systematic names for the following complexes: K3[Fe(CN)6]; [Cr(NH3)6]Br3 Give the structures for the following compounds: sodium fac–trichlorotrifluorocobaltate(III); dicyano–η2–etheneplatinum(II).
K3[Fe(CN)6]: potassium hexacyanoferrate(III)
[Cr(NH3)6]Br3: hexaamminechromium(III) bromide
sodium fac–trichlorotrifluorocobaltate(III):
dicyano–η2–etheneplatinum(II):
4. Find the ligand field stabilization energy in terms of Dq for the following complexes: Cr(NH3)63+; Fe(NH3)62+; Cr(CN)63–; Zn(NH3)42+. Order the complexes from least stable to most stable.
Cr(NH3)63+: d3 in an Oh field so LFSE = –12Dq
Fe(NH3)62+: d6 in an Oh field with a weak ligand so LFSE = –4Dq
Cr(CN)63–: d3 in an Oh field so LFSE = –12Dq
Zn(NH3)42+: d10 so LFSE = 0.
The stability based on LFSE is Zn(NH3)42+ < Fe(NH3)62+ < Cr(NH3)63+ < Cr(CN)63–
(The cyano complex is more stable than the ammine complex because Dq is larger for the stronger ligand CN–.)/p>
5. Which of the following compounds is stable as judged by the effective atomic number rule? Ni(CO)4; V(CO)6; Mn(cp)2; Cu(NH3)2Cl2. For those that you predict to be unstable, what is a likely chemical reaction for the compound? cp = η5–C5H5–
Ni(CO)4: Ni0 is d10, each CO contributes 2 e– so the electron count is 10 + 4(2) = 18, stable.
V(CO)6: V0 is d5, each CO contributes 2 e– so the electron count is 5 + 6(2) = 17, unstable; expect reduction to V(CO)6– to obtain the stable 18 electron configuration. (In the solid state, V(CO)6 exists as ions: [V(CO)6]+[V(CO)6]– to increase stability.)
Mn(cp)2: Mn2+ is d5, each cp ring contributes 6 e– so the electron count is 5 + 2(6) = 17, unstable; expect reduction to Mn(cp)2–.
Cu(NH3)2Cl2: Cu2+ is d9, each NH3 contributes 2 e–, each Cl– contributes 2 e– so the electron count is 9 + 2(2) + 2(2) = 17, unstable by EAN. Although the EAN rule is violated, Cu2+ is more ionic than covalent so the EAN rule is not a good guide so no prediction about reactivity is justified.