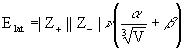Chemistry 401
Intermediate Inorganic Chemistry
University of Rhode Island
Fall 1999
Final Exam
All references are to papers from Inorg. Chem., 1999, 38.
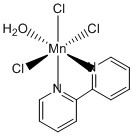
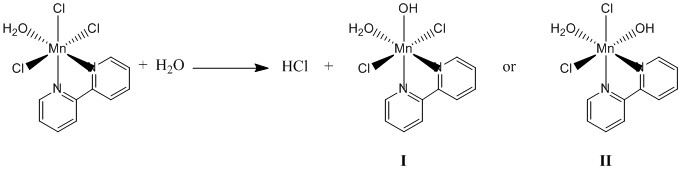
1. C. Philouze, M. Henry, A. Auger, D. Vignier, M. Lance, M. Nierlich, and J.-J. Girerd (p. 4-11) examined some properties of fac-aqua(2,2'-bipyridine)trichloromanganese(III). Draw the structure of the compound. This compound may react with water to eliminate HCl in an acid-base reaction. The product complex maintains a coordination number of six but may have two isomers. Write the reaction, draw structures for the two isomers, and name them.
The structure of fac-aqua(2,2'-bipyridine)trichloromanganese(III) is
The proposed reaction is:
I = aqua(2,2'-bipyridine)-trans-bichloromanganese(III)
II = aqua(2,2'-bipyridine)-cis-bichloromanganese(III)
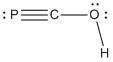
2. K. Hübler and P. Schwerdtfeger (p. 157-164) considered the properties of PCOH. Draw the Lewis dot structure, estimate the bond angles about the C and O atoms, and give the point group for the molecule.
Lewis structure:
P-C-O angle = 180°
C-O-H angle ~ 107°
Point Group: Cs
3. P. D. Metelski and T. W. Swaddle (p. 301-307) studied the self-exchange reaction
[Os(CN)6]3– + [Os(CN)6]4– → [Os(CN)6]4– + [Os(CN)6]3–
Name each complex. The rate constant for this reaction was found to be 1.4×10–4 L mol–1s–1. However, when (CH3)4N+ ion was added to the solution, the exchange rate increased by a factor of about 100. In the absence of tetramethylammonium ion, is the self-exchange inner-sphere or outer-sphere? How does addition of the tetramethylammonium change the reaction?
[Os(CN)6]3–: hexacyanoosmate(III) ion
[Os(CN)6]4–: hexacyanoosmate(II) ion
The self exchange is likely outer sphere since there are no open coordination sites for bridging to occur. The large cation allows the two anions to get closer to each other via Coulomb attraction to the cation, acting to screen the negative charge between the two anions. This allows the electron transfer rate to increase.
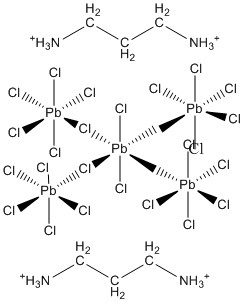
4. A. B. Corradi, A. M. Ferrari, G. C. Pellacani, A. Saccani, F. Sandrolini, and P. Sgarabotto (p. 716-721) reported the structure of (H3N+-CH2CH2CH2NH3+)(PbCl4). The compound is not closest packed; rather a layered structure is found. One of the layers is formed by chains of PbCl6 octahedra; draw a sketch of this (remember to maintain the observed stoichiometry). The compound was found to be a reasonably good conductor of electricity, but the charge carriers are ions rather than electrons. Which ion is likely to be the most mobile, thus responsible for the electrical conductivity?
H+ is the most mobile since it light and can exchange sites via H-bonding.
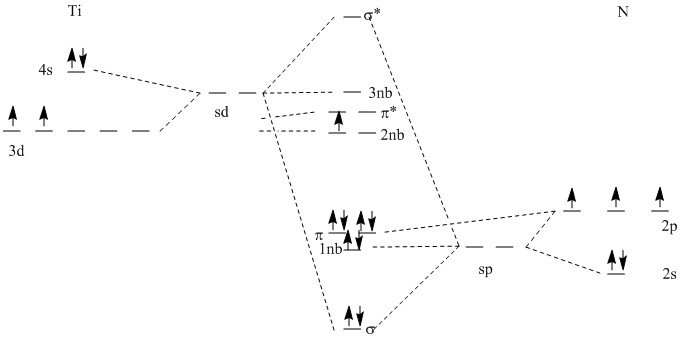
5. J. Cheon, M. Guile, P. Maraoka, and J. I. Zink (p. 2238-2239) looked at the gas phase decomposition of Ti(N(CH2CH3)4)4 and found TiN as one of the gas phase products. TiN was identified spectroscopically by an emission in the visible region at about 616 nm (a fairly low energy). Draw a simple MO diagram for TiN. For a basis set, use the 4s and 3d orbitals on Ti and the 2s and 2p orbitals on N and assume the appropriate sp or sd hybridization occurs. What is the bond order?
B.O. = 3 (σ and two π orbitals are occupied. The remaining electrons are in nonbonding orbitals.)
6. M. Scholten, R. Dronskowski, and H. Jacobs (p. 2614-2620) reacted InBr3 and Cr metal to give a product of InCrBr3. The product was found to have a room temperature magnetic moment of 4.83 μB. What are the oxidation states of In and Cr in the product? The geometry about the Cr is distorted and this was attributed to a Jahn-Teller effect. What type of distortion would you expect?
A magnetic moment of 4.83 μB suggests 4 unpaired electrons, which means that the Cr is in the +2 (d4, weak field, high spin) state. Since Br must be –1, this requires than In be in the +1 oxidation state.
A d4, high spin state has an electron configuration of t2g3eg1 configuration, which can distort either by elongation or contraction. No prediction is possible.
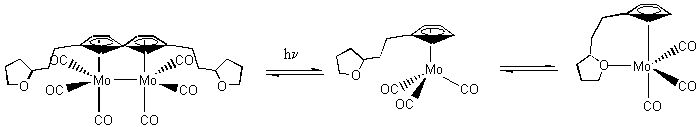
7. M. Gallagher, P. Dougherty, P. S. Tanner, D. C. Barbini, J. Schulte, and W. E. Jones, Jr. (p. 2953-2956) found an unusual reaction sequence, shown below.
What is the electron count about the Mo in each species? What about the reaction sequence should be considered unusual?
In the reactant, each Mo is in the +1 oxidation state so is d5. Each CO contributes 2 e– for a total of 6 e–. The cp– ring contributes 6 e–. Finally, the Mo-Mo- bond contributes 1 e–. The total is 5 + 6 + 6 + 1 = 18 for each Mo (a stable complex).
The photoproduct intermediate has Mo+ [5 e–] + 3 CO [3×2 e–] + cp– [6 e–] = 17 e–
The product has Mo+ [5 e–] + 3 CO [6 e–] + cp– [6 e–] + 1 ether oxygen [2 e–] = 19 e–
The product is a 19 e– species, which is expected to be unstable, which makes this reaction unexpected.
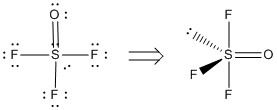
8. A. Kornath, D. Kadzimirsz, and R. Ludwig (p. 3066-3069) synthesized trifluorosulfite ion, SOF3–. Draw the Lewis structure, predict the bond angles, and give the point group.
F-S-F angles: ~178° and ~88°
F-S-O angles: ~118° and ~88°
Point Group: Cs
9. H. D. B. Jenkins, H. K. Roobottom, J. Passmore, and L. Glasser (p. 3609-3620) devised a new way to estimate lattice energies for ionic salts, MpXq. They give the working equation as
where ν is the number of ions per molecule (= p + q), Z+ and Z– are the ionic charges, V is the ionic volume in nm3, and α = 138.7 kJ/mol and β = 27.6 kJ/mol. The ionic volume is found from V = pV+ + qV– and V+ and V– are found from ionic radii (V = 4πr3/3). Use this formula to estimate the lattice energy for NaCl using the data in Table 1.5. Comment on the reliability of this method.
rNa+ = 102 pm = 0.102 nm and rCl– = 181 pm = 0.181 nm
Thus, V+ = 4(3.14159)(0.102)3/3 = 5.28×10–3 nm3 and V– = 4(3.14159)(0.181)3/3 = 2.48×10–2 nm3
Then, V = (1)(5.28×10–3) + (1)(2.48×10–2) = 3.01×10–2 nm3
Elat = |+1||–1|(1+1)(138.7/∛(3.01×10–2)+27.6) = 947 kJ/mol
This overestimates the lattice energy considerably, suggesting that the method is only semi-quantitative, at best.
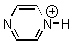
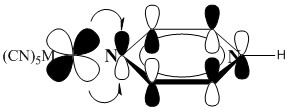
10. L. D. Slep, S. Pollack, and J. A. Olabe (p. 4369-4371) measured the pKa values for [(NC)5M(pyrH)]2– where M = Fe(II) or Ru(II) and pyrH is shown below. They attribute the difference in acidity to backbonding into the pyrazine π* orbitals. Explain how the backbonding can affect the pKa of the remote H atom. Predict which metal will give a stronger acid.
pyrH =
The backbonding occurs from a metal dπ (dxz or dyz) into an aromatic π* orbital:
The addition of electron density onto the pyrazine ring reduces the positive charge on the nitrogen atom, which will decrease the H+ donation ability. Since Ru(II) will have a larger Z*, less electron density will be donated into the pyrazine ring, which will increase the H+ donation, which will lead to a lower pKa.