Chemistry 401
Intermediate Inorganic Chemistry
University of Rhode Island
Fall 1999
Exam 3
1. Does ΔH° calculated using the Drago/Wayland equation agree with the Donor Number for the listed bases? Why or why not? Suppose trimethyltin chloride had been used as the standard for the DN scale; would this change the order of base strength for the listed bases? Why or why not?
2. Use the information in the Latimer diagram to construct the Frost diagram for iron in acidic solution. Use the provided graph paper and label each axis.
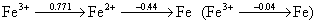
3. [Ru(bipy)3]3+ is a strong oxidizing agent that nearly always undergoes electron transfer by an outer sphere mechanism. Why is the mechanism outer sphere? What kinetic advantages might this oxidizing agent possess over a weaker oxidizing agent?
4. Balance the following reaction under acidic conditions:
CrO42–(aq) + H2SO3(aq) → Cr3+(aq) + HSO4–(aq)
5. Name the following: a) [Fe(NH3)5Cl]SO4
b) 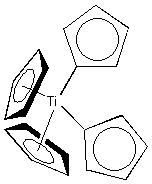
6. Give the structure for mer-triamminebromo-cis-dichlorocobalt(III).
7. Consider hexacyanochromate(III) and hexaaquachromium(III) ions. Find the LFSE for each; which will be larger? Estimate the spin-only magnetic moment for each ion in units of Bohr-Magnetons.
