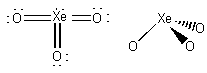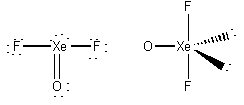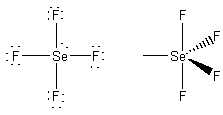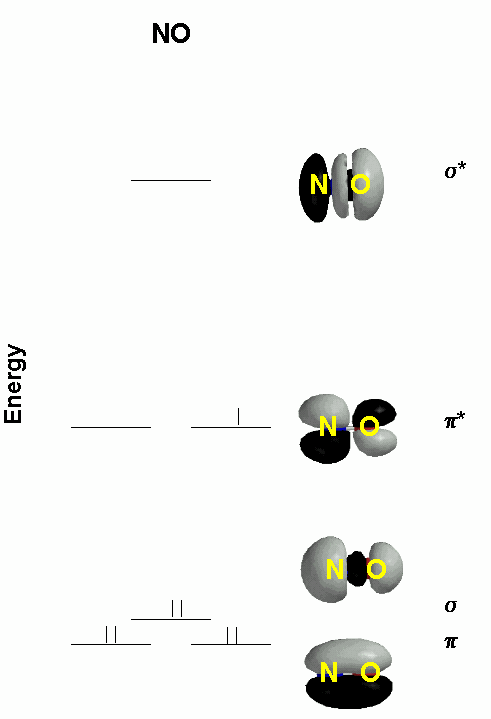Chemistry 401
Intermediate Inorganic Chemistry
University of Rhode Island
Fall 1999
Exam 2
1. For each of the following species write the lowest energy Lewis structure, indicate the formal charge and oxidation number of each atom, show the geometric structure and estimate the bond angles, indicate the σ hybridization about the central atom, and give the point group. a) XeO3, b) XeOF2, c) SeF4.
a)
formal charges: O, 0; Xe, 0.
oxidation numbers: O, -2; Xe, +6.
geometric structure: pyramidal, O-Xe-O angles ~108°
Xe hybridization: sp3
point group: C3v
b)
formal charges: O, 0; Xe, 0; F, 0.
oxidation numbers: O, -2; Xe, +4; F, -1.
geometric structure: bent-T, O-Xe-F angles ~89°
Xe hybridization: dsp3
point group: C2v
c)
formal charges: F, 0; Se, 0.
oxidation numbers: F, -1; Se, +4.
geometric structure: see-saw, F-Se-F angles of ~118° and ~88°
Se hybridization: dsp3
point group: C2v
2. Consider the molecular orbital diagram for NO, given below. This was constructed using only 2p orbitals as the basis set. Label the type of orbital (σ, π, δ, bonding, antibonding) at each energy level, show the orbital occupation, and find the bond order. Compare this to a Valence Bond Theory explanation of the bonding. What are the bond orders for NO+ and NO–; would you expect these ions to be stable? Why or why not?
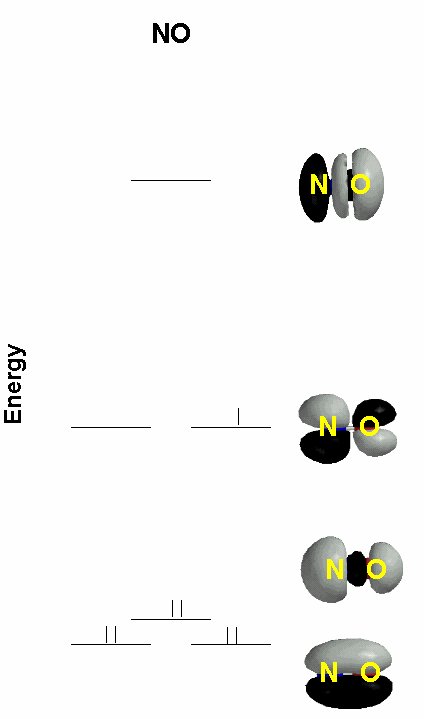
NO Bond Order = ½(6-1) = 2.5
VBT
This has a double bond with the unpaired spin localized on the nitrogen, compared to the B.O. = 2.5 and the unpaired spin delocalized over both atoms in the *pi;* orbital in MO theory. The simple MO theory also does not have well defined lone pairs, although the σ orbital has a lot of density pointing away from the N and O atoms.
NO+: B.O. = 3; NO–: B.O. = 2; Both are expected to be stable because of the high bond orders.
3. Mg is a metal, yet has a filled s orbital. Explain, in terms of band theory and appropriate atomic properties, why Mg can attain a partially filled band to give it metallic properties.
The electron configuration for Mg is [Ne]3s23p0 and Z* is 3.3. As the bands begin to form, the energies of both the s and p orbitals begin to spread out and since Z* is of moderate value, the p orbital energies are close to the s orbital energies. This means that upon band formation the top of the s-band is above the bottom of the p-band, i.e., the bands overlap in energy. This allows electrons from the s-band to occupy the p-band, leaving both partially occupied, which leads to the metallic character.