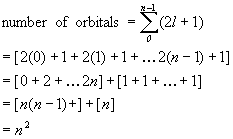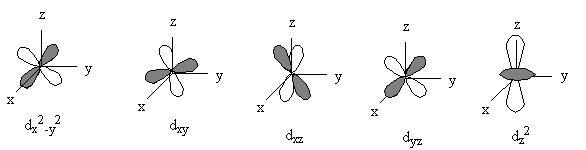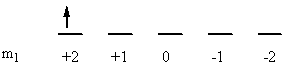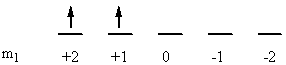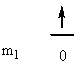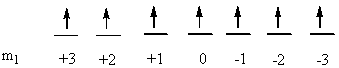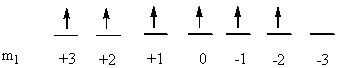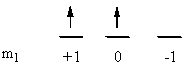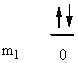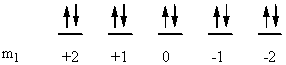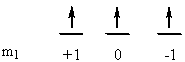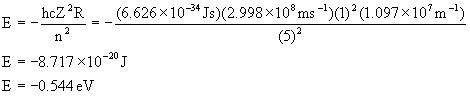Chemistry 401
Intermediate Inorganic Chemistry
University of Rhode Island
Practice Problems
Atomic Structure and Periodic Properties
1. How many orbitals are there in a shell of principal quantum number n? (Hint: begin with n = 1, 2, and 3 and see if you can recognize the pattern.)
Starting with the hint:
n = 1 gives 1 orbital (1s)
n = 2 gives 4 orbitals (2s, 2p)
n = 3 gives 9 orbitals (3s, 3p, 3d)
in general, the pattern gives n2 orbitals.
More rigorously, for each n there are l values of n–1, ..., 0 and for each l value, ml = –l, –l+1, ..., l, or 2l + 1 orbitals. Summing these for all l values gives
2. a) On a single graph, sketch the radial wavefunctions for the 4f and 5f orbitals. b) On a single graph, sketch the radial probability functions for the 4f and 5f orbitals. c) Sketch all of the angular wavefunctions for the 4d orbitals.
a) On a single graph, sketch the radial wavefunctions for the 4f and 5f orbitals.
b) On a single graph, sketch the radial probability functions for the 4f and 5f orbitals.
c) Sketch all of the angular wavefunctions for the 4d orbitals.
3. Give the ground-state electron configurations and ground state term symbol of (a) Sc, (b) V3+, (c) Mn2+, (d) Cr2+, (e) Co3+, (f) Cr6+, (g) Cu, (h) Gd3+.
(a) Sc: [Ar]4s23d1
L = 2 (D state)
S = ½ so 2S + 1 = 2(½) + 1 = 2
term = 2D
(b) V3+: [Ar]3d2
L = 2 + 1 = 3 (F state)
S = ½ + ½ = 1 so 2S + 1 = 2(1) + 1 = 3
term = 3F
(c) Mn2+: [Ar]3d5
L = 2 + 1 + 0 + –1 + –2 = 0 (S state)
S = ½ + ½ + ½ + ½ + ½ = 5/2 so 2S + 1 = 2(5/2) + 1 = 6
term = 6S
(d) Cr2+: [Ar]3d4
L = 2 + 1 + 0 + –1 = 2 (D state)
S = ½ + ½ + ½ + ½ = 2 so 2S + 1 = 2(2) + 1 = 5
term = 5D
(e) Co3+: [Ar]3d6
L = 2 + 2 + 1 + 0 + -1 = 2 (D state)
S = ½ + ½ + ½ + ½ = 2 so 2S + 1 = 2(2) + 1 = 5
term = 5D
(f) Cr6+: [Ar]
filled shell, so L = 0 (S state) and S = 0 so 2S + 1 = 2(0) + 1 = 1
term = 1S
(g) Cu: [Ar]4s13d10 (this is one of the exceptions from the Periodic Table using the half-filled rule)
L = 0 (S state)
S = ½ so 2S + 1 = 2(½) + 1 = 2
term = 2S
(h) Gd3+: [Xe]4f7
L = 3 + 2 + 1 + 0 + –1 + –2 + –3 = 0 (S state)
S = ½ + ½ + ½ + ½ + ½ + ½ + ½ = 7/2 so 2S + 1 = 2(7/2) + 1 = 8
term = 8S
4. Give the ground-state electron configuration and ground state term symbol of (a) W, (b) Rh3+, (c) Eu3+, (d) Eu2+, (e) V5+, (f) Mo4+.
(a) W: [Xe]6s24f145d4 (This one does not go to half-filled, oddly enough)
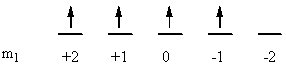
L = 2 + 1 + 0 + –1 = 2 (D state)
S = ½ + ½ + ½ + ½ = 2 so 2S + 1 = 2(2) + 1 = 5
term = 5D
(b) Rh3+: [Kr]4d6
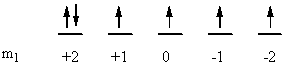
L = 2 + 2 + 1 + 0 + –1 = 2 (D state)
S = ½ + ½ + ½ + ½ = 2 so 2S + 1 = 2(2) + 1 = 5
term = 5D
(c) Eu3+: [Xe]4f6
L = 3 + 2 + 1 + 0 + –1 + –2 = 3 (F state)
S = ½ + ½ + ½ + ½ + ½ + ½ = 3 so 2S + 1 = 2(3) + 1 = 7
term = 7F
(d) Eu2+: [Xe]4f7
L = 3 + 2 + 1 + 0 + –1 + –2 + –3 = 0 (S state)
S = ½ + ½ + ½ + ½ + ½ + ½ + ½ = 7/2 so 2S + 1 = 2(7/2) + 1 = 8
term = 8S
(e) V5+: [Ar]
filled shell so L = 0 (S state) and S = 0 so 2S + 1 = 2(0) + 1 = 1
term = 1S
(f) Mo4+: [Kr]4d2
L = 2 + 1 = 3 (F state)
S = ½ + ½ = 1 so 2S + 1 = 2(1) + 1 = 3
term = 3F
5. Give the ground-state electron configuration and ground state term symbol of (a) C, (b) F, (c) Ca, (d) Ga3+, (e) Bi, (f) Pb2+.
(a) C: [He]2s22p2
L = 1 + 0 = 1 (P state)
S = ½ + ½ = 1 so 2S + 1 = 2(1) + 1 =3
term = 3P
(b) F: [He]2s22p5
L = 1 + 1 + 0 + 0 + -1 = 1 (P state)
S = ½ + ½ + ½ + –½ + –½ = ½ so 2S + 1 = 2(½) + 1 = 2
term = 2P
(c) Ca: [Ar]4s2
L = 0 + 0 = 0 (S state)
S = ½ + –½ = 0 so 2S + 1 = 2(0) + 1 = 1
term = 1S
(d) Ga3+: [Ar]3d10
L = 2+ 2+ 1 + 1 + 0 + 0 + –1 + –1 + –2 + –2 = 0 (S state)
S = ½ + –½ + ½ + –½ + ½ + –½ + ½ + –½ + ½ + –½ = 0 so 2S + 1 = 2(0) + 1 = 1
term = 1S
(e) Bi: [Xe]6s25d104f146p3
L = 1 + 0 + –1 = 0 (S state)
S = ½ + ½ + ½ = 3/2 so 2S + 1 = 2(3/2) + 1 = 4
term = 4S
(f) Pb2+: [Xe]6s25d104f14
filled shell, so L = 0 (S state) and S = 0 so 2S + 1 = 2(0) + 1 = 1
term = 1S
6. Find the electron configuration and ground state term symbol for the following species: Cl, Cl–, Mo, Mo+, Mo2+, Mo3+.
SpeciesElectron ConfigurationOrbital Occupation L, STerm
Cl[Ne]3s23p5
L = 1, S = 1/22P
Cl–[Ar]Closed Shell L = 0, S = 01S
Mo[Kr]5s14d5
L = 0, S = 37S
Mo+[Kr]4d5
L = 0, S = 5/26S
Mo2+[Kr]4d4
L = 2, S = 25D
Mo3+[Kr]4d3
L = 3, S = 3/24F
7. Write the electron configuration and give the ground state term symbol for: a) Fe; b) Fe2+; c) Ir+; d) Ir3+; and e) I–.
a) Fe: [Ar]4s23d6
↑ ↓ ↑ ↓ ↑ ↑ ↑ ↑
ml0+2 +10 –1–2
So L = 0 + 0 + 2 + 2 + 1 + 0 + –1 + –2 = 2 (D state) and S = ½ + –½ + ½ + –½ + ½ + ½ + ½ + ½ = 2 (2S + 1 = 5). This gives a term symbol of 5D.
b) Fe2+: [Ar]3d6
↑ ↓ ↑ ↑ ↑ ↑
ml+2 +10 –1–2
So L = 2 + 2 + 1 + 0 + –1 + –2 = 2 (D state) and S = ½ + –½ + ½ + ½ + ½ + ½ = 2 (2S + 1 = 5). This gives a term symbol of 5D.
c) Ir+: [Xe]4f145d8
↑ ↓ ↑ ↓ ↑ ↓ ↑ ↑
ml+2 +10 –1–2
So L = 2 + 2 + 1 + 1 + 0 + 0 + –1 + –2 = 3 (F state) and S = ½ + –½ + ½ + –½ + ½ + –½ + ½ + ½ = 1 (2S + 1 = 3). This gives a term symbol of 3F.
d) Ir3+: [Ar]4f145d6
↑ ↓ ↑ ↑ ↑ ↑
ml+2 +10 –1–2
So L = 2 + 1 + 0 + –1 + –2 = 2 (D state) and S = ½ + –½ + ½ + ½ + ½ + ½ = 2 (2S + 1 = 5). This gives a term symbol of 5D.
e) I–: [Xe]
This is a closed shell ion so L = 0 (S state) and S = 0 (2S + 1 = 1). This gives a term symbol of 1S.
8. Write the electron configuration and give the ground state term symbol for: a) Ni; b) Ni2+; c) Rh+; d) Rh3+; and e) S2–.
a) Ni: [Ar]4s23d8
↑ ↓ ↑ ↓ ↑ ↓ ↑ ↓ ↑ ↑
ml0+2 +10 –1–2
So L = 0 + 0 + 2 + 2 + 1 + 1 + 0 + 0 + –1 + –2 = 3 (F state) and S = ½ + –½ + ½ + –½ + ½ + –½ + ½ + –½ + ½ + ½ = 1 (2S + 1 = 3). This gives a term symbol of 3F.
b) Ni2+: [Ar]3d8
↑ ↓ ↑ ↑ ↑ ↑
ml+2 +10 –1–2
So L = 2 + 2 + 1 + 1 + 0 + 0 + –1 + –2 = 3 (F state) and S = ½ + –½ + ½ –½ + ½ –½ + ½ + ½ = 1 (2S + 1 = 3). This gives a term symbol of 3F.
c) Rh+: [Kr]4d8
↑ ↓ ↑ ↓ ↑ ↓ ↑ ↑
ml+2 +10 –1–2
So L = 2 + 2 + 1 + 1 + 0 + 0 + –1 + –2 = 3 (F state) and S = ½ + –½ + ½ + –½ + ½ + –½ + ½ + ½ = 1 (2S + 1 = 3). This gives a term symbol of 3F.
d) Rh3+: [Kr]4d6
↑ ↓ ↑ ↑ ↑ ↑
ml+2 +10 –1–2
So L = 2 + 1 + 0 + –1 + –2 = 2 (D state) and S = ½ + –½ + ½ + ½ + ½ + ½ = 2 (2S + 1 = 5). This gives a term symbol of 5D.
e) S2–: [Ar]
This is a closed shell ion so L = 0 (S state) and S = 0 (2S + 1 = 1). This gives a term symbol of 1S.
9. Compare the first ionization energy of calcium with that of zinc. Explain the difference in terms of the balance between shielding with increasing numbers of d electrons and the effect of increasing nuclear charge.
Ca [Ar]4s2 IP = 6.113 eV
Zn [Ar]3d104s2 IP = 9.394 eV
Both are ionizing the outer 4s electron but the zinc IP is significantly higher. This is a result of the higher nuclear charge for Zn (Z = 30) being poorly shielded by the 3d electrons. Since the operative parameter is Z* = Z – S, Z increases by 10 for Zn compared to Ca and S increases less than 10 units, perhaps only 7 or 8 units. Thus, Z* for Zn is much higher, leading to a higher ionization energy.
10. Compare the first ionization energies of strontium, barium, and radium. Relate the irregularity to the lanthanide contraction.
Sr IP = 6.595 eV
Ba IP = 5.212 eV
Ra IP = 5.278 eV
The ionization energy decreases from Sr to Ba because the principal quantum number increases by 1, leading to a larger valence orbital and consequent easier ionization. The change from Ba to Ra is almost nothing, even though there has been another increase of 1 in n. This arises because Ra has a filled 4f subshell; the 4f electrons shield very poorly (three nodes at the nucleus) so that Z* for Ra is higher than might be otherwise anticipated. The higher effective charge available for the Ra valence electrons causes a contraction of the 7s orbital and an increase of the ionization energy, which would have been predicted to be around 4 eV in the absence of the lanthanide contraction.
11. The second ionization energy of some Period 4 elements are
ElementIE (kJ/mol)
Ca1145
Sc1235
Ti1310
V1365
Cr1592
Mn1506
Identify the orbital from which ionization occurs and account for the trend in the values.
Electron configurations:
Ca [Ar]4s2Ca+ [Ar]3d1 Ca2+ [Ar]
Sc [Ar]4s23d1Sc+ [Ar]3d2 Sc2+ [Ar]3d1
Ti [Ar]4s23d2Ti+ [Ar]3d3 Ti2+ [Ar]3d2
V [Ar]4s23d3V+ [Ar]3d4 V2+ [Ar]3d3
Cr [Ar]4s13d5Cr+ [Ar]3d5 Cr2+ [Ar]3d4
Mn [Ar]4s23d5Mn+ [Ar]3d6 Mn2+ [Ar]3d5
The Second Ionization always occurs from the 3d orbital. Ionization from Cr+ is unusually high because ionization occurs from a half-filled d subshell while ionization from Mn+ is a bit low because the product ion Mn2+ is half-filled.
12. The ionization energies of rubidium and silver are respectively 4.18 eV and 7.57 eV. Calculate the ionization energies of an H atom with its electron in the same orbitals as in these two atoms and account for the differences in values.
Rb [Kr]5s1
Ag [Kr]4d105s1
For both atoms, ionization occurs from the 5s orbital, so:
Thus, the calculated ionization energy is 0.544 eV and is the same for both Rb and Ag. The observed values are quite different from the calculated one because of incomplete shielding of the core electrons so that the 5s electron feels a charge greater than +1 for both Rb and Ag. The two atoms are different because the 4d electrons screen the nuclear charge relatively poorly, giving a higher Z* for Ag.
13. In general, ionization energies increase across a period from left to right. Explain why the second ionization energy of chromium is higher, not lower, than that of manganese.
The reactions associated with the second ionization potentials are:
Cr+(g) → Cr2+(g) + e– (IP2 for Cr)
and
Mn+(g) → Mn2+(g) + e– (IP2 for Mn)
The electron configurations for each species is:
Cr+: [Ar]3d5 → Cr2+: [Ar]3d4
Mn+: [Ar]3d6 → Mn2+: [Ar]3d5
In the case of Cr, the reactant is half-filled, imparting extra stability and requiring more energy for ionization. In the case of Mn, the product is half-filled, giving extra stability to the product and requiring less energy for ionization. The two effects together lead to IP2(Cr) > IP2(Mn).
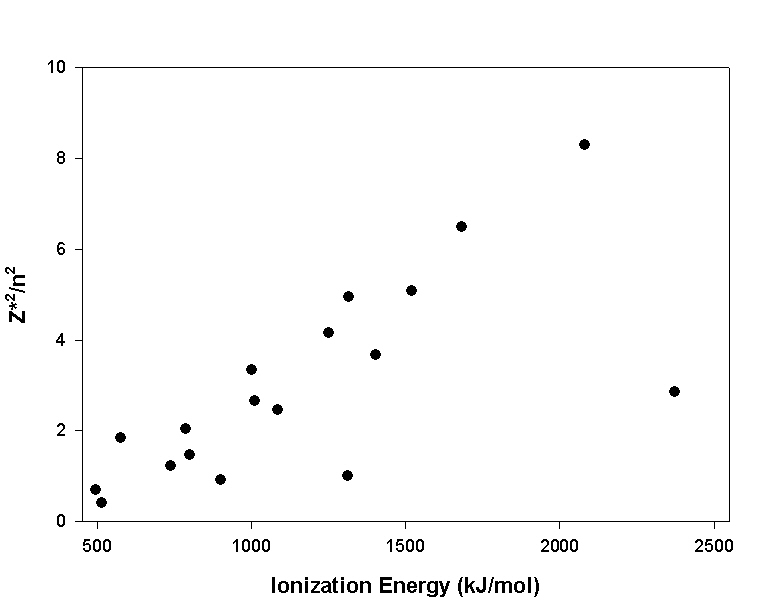
14. It has been proposed that the ionization energy is proportional to Z*2/n2, where Z* is the effective nuclear charge and n is the principal quantum number. Make a plot of IE (x-axis) vs. Z*2/n2 (y-axis). Is the assertion justified? Why or why not? Are there exceptions to the generalization? If so, what are those exceptions and can you rationalize them?
The plot of the data is given below:
The plot is generally linear, which supports the proposed idea. There are two prominent points that lie well off the main linear region: H (IE = 1312 kJ/mol, Z*2/n2 = 1.0) and He (IE = 2373 kJ/mol, Z*2/n2 = 2.9). It seems that shielding from the core electrons is essential for this proportionality to work. Since both H and He have no core electrons, they do not fit the generalization.
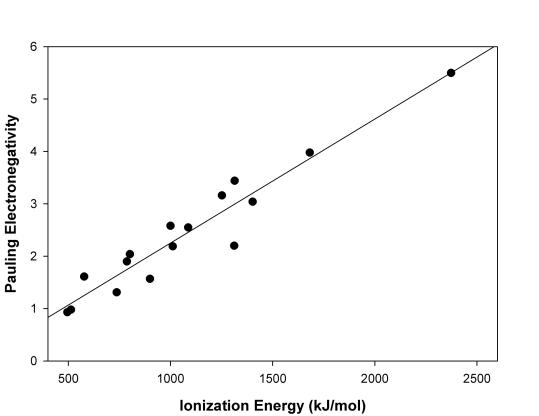
15. Make a plot of IE vs. χ (χ = Pauling electronegativity) for the elements 1 through 18. Does your graph suggest any relationship between the two quantities? Why or why not?
The plot of the data is given below:
The plot is generally linear. This suggests that electronegativity (a defined quantity) may depend upon ionization energy (a measured quantity). Indeed, this is the basis for the Mulliken definition of electronegativity.
16. Account for the fact the two Group 5 elements niobium (Period 5) and tantalum (Periodic 6) have the same metallic radii.
Look at the electron configurations:
Nb: [Kr]5s24d3
Ta: [Xe]6s24f145d3
The presence of the f electrons is the difference: the f orbitals do a very poor job of shielding the valence electrons from the nucleus (because of the three angular nodes at the nucleus) so the effective nuclear charge of Ta is higher than that of Nb. Thus, even though the valence electrons of Ta are in orbitals with larger principal quantum number, the extra Z* contracts the orbitals to be the same size as Nb. This is known as the lanthanide contraction.
17. Consider the process of shielding in atoms, using Be as an example. What is being shielded? What is it being shielded from? What is doing the shielding?
Using Be as an example, the electron configuration is 1s22s2. The valence electrons (those in the 2s orbital) are being shielded from the nuclear charge. This shielding is done by the 1s electrons, which are somewhat interior to the 2s electrons, at least on average, and overlap significantly with the nucleus.
18. Compare the broad trends in ionization energy, atomic radius, and electronegativity. Account for the parallels.
Moving from left to right on the Periodic Table, the ionization potential and electronegativity increase and the atomic radius (no matter how it is measured) decreases. The reverse trend is observed going down the Periodic Table: the ionization potential and the electronegativity decrease and the atomic radius increases. The trends all follow Z*, which increases from left to right because of poor shielding as electrons fill orbitals of the same principal quantum number, n. Increasing Z* implies a stronger attraction for electrons to the nucleus, so IP and χ increase and the radius decreases. Going down the Periodic Table Z* slightly decreases because of increasing principal quantum number, n.