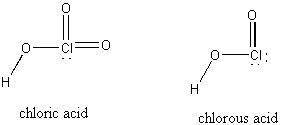Chemistry 401
Intermediate Inorganic Chemistry
University of Rhode Island
Practice Problems
Acids & Bases
1. Identify the conjugate bases corresponding to the following acids: [Co(NH3)5(OH2)]3+, HSO4–, CH3OH, H2PO4–, Si(OH)4, HS–. Also write the acid-base reaction with water for each acid and label the acid, base, and conjugate acid.
[Co(NH3)5(OH2)]3+(aq) + H2O(l) →← [Co(NH3)5(OH)]2+(aq) + H3O+(aq)
Acid: [Co(NH3)5(OH2)]3+
Base: H2O
Conjugate acid: H3O+
Conjugate base: [Co(NH3)5(OH)]2+
HSO4–(aq) + H2O(l) → ← SO42–(aq) + H3O+(aq)
Acid: HSO4–
Base: H2O
Conjugate acid: H3O+
Conjugate base: SO42–
CH3OH(aq) + H2O(l) → ← CH3O–(aq) + H3O+(aq)
Acid: CH3OH
Base: H2O
Conjugate acid: H3O+
Conjugate base: CH3O–
H2PO4–(aq) + H2O(l) → ← HPO42–(aq) + H3O+(aq)
Acid: H2PO4–
Base: H2O
Conjugate acid: H3O+
Conjugate base: HPO42–
Si(OH)4(aq) + H2O(l) → ← SiO(OH)3–(aq) + H3O+(aq)
Acid: Si(OH)4
Base: H2O
Conjugate acid: H3O+
Conjugate base: SiO(OH)3–
HS–(aq) + H2O(l) → ← S2–(aq) + H3O+(aq)
Acid: HS–
Base: H2O
Conjugate acid: H3O+
Conjugate base: S2–
2. Identify the conjugate acids of the bases C5H5N (pyridine), HPO42–, O2–, CH3COOH, [Co(CO)4]–, CN–. Also write the acid-base reaction with water for each base and label the acid, base, and conjugate base.
C5H5N(aq) + H2O(l) → ← C5H5NH+(aq) + OH–(aq)
Acid: H2O
Base: C5H5N
Conjugate acid: C5H5NH+
Conjugate base: OH–
HPO42–(aq) + H2O(l) → ← H2PO4–(aq) + OH–(aq)
Acid: H2O
Base: HPO42–
Conjugate acid: H2PO4–
Conjugate base: OH–
O2–(aq) + H2O(l) → 2 OH–(aq)
Acid: H2O
Base: O2–
Conjugate acid: OH–
Conjugate base: OH–
CH3COOH(aq) + H2O(l) → ← CH3COOH2+(aq) + OH–(aq)
Acid: H2O
Base: CH3COOH
Conjugate acid: CH3COOH2+
Conjugate base: OH–
[Co(CO)4]–(aq) + H2O(l) → ← [HCo(CO)4](aq) + OH–(aq)
Acid: H2O
Base: [Co(CO)4]–
Conjugate acid: [HCo(CO)4]
Conjugate base: OH–
CN–(aq) + H2O(l) → ← HCN(aq) + OH–(aq)
Acid: H2O
Base: CN–
Conjugate acid: HCN
Conjugate base: OH–
3. The ions Na+ and Ag+ have similar radii. Which aqua ion is the stronger acid? Why?
Ag+ has a higher Z* because of the poor shielding of d electrons. The higher positive charge density on the silver ion implies that the aqua ion, [Ag(H2O)n]+, will more strongly repel an H+ ion. Thus, Ag+ is the stronger acid.
4. Predict whether the equilibrium constants for the following reactions should be greater than 1 or less than 1:
(a) CdI2(s) + CaF2(s) → ← CdF2(s) + CaI2(s)
(b) [CuI4]2–(aq) + [CuCl4]3–(aq) → ← [CuCl4]2–(aq) + [CuI4]3–(aq)
(c) NH2–(aq) + H2O(l) → ← NH3(aq) + OH–(aq)
(a) Use hard-soft acid base theory to approach the problem:
CdI2(s)+CaF2(s) →← CdF2(s)+CaI2(s)
SA–SBHA–HB SA–HBHA–SB
The preferred direction is reactants so the equilibrium constant is less than 1.
(b) Again, use hard-soft acid base theory to approach the problem:
[CuI4]2–(aq)+ [CuCl4]3–(aq) →← [CuCl4]2–(aq)+ [CuI4]3–(aq)
HA–SBSA–HB HA–HBSA–SB
The preferred direction is products so the equilibrium constant is greater than 1.
(c) Since the acid portion is the same in each of these, HSAB theory is less useful. Recall that water can either donate or accept a hydrogen ion in water but ammonia is a very poor hydrogen ion donor, meaning that amide ion is a very strong base:
NH2–(aq) + H2O(l) → ← NH3(aq) + OH–(aq)
The preferred direction is products so the equilibrium constant is greater than 1.
5. Give the equation for the dissolution of SiO2 glass by HF and interpret the reaction in terms of Lewis and Brønsted acid-base concepts.
SiO2 + 4 HF → SiF4 + 2 H2O
Lewis interpretation:
Acid: HF
Base: SiO2
New covalent bonds: Si–F and O–H
Brønsted-Lowry interpretation:
Acid: HF
Base: SiO2
Conjugate Acid: H2O
Conjugate Base: SiF4
6. For parts (a), (b), and (c) state which of the two solutions has the lower pH:
(a) 0.1 M Fe(ClO4)2 or 0.1 M Fe(ClO4)3
(b) 0.1 M Ca(NO3)2 or 0.1 M Mg(NO3)2
(c) 0.1 M Hg(NO3)2 or Zn(NO3)2
In all cases the concentration of the two solutions to be compared is the same, so the decision about lower pH relies solely on the relative acidity of each component.
(a) 0.1 M Fe(ClO4)3 or 0.1 M Fe(ClO4)3
Fe3+ has a higher charge than Fe2+ so will withdraw more electron density from the OH bonds in the water coordinated to the ion in aqueous solution. Thus, Fe3+ will be more acidic and have the lower pH.
(b) 0.1 M Ca(NO3)2 or 0.1 M Mg(NO3)2
Ca2+ and Mg2+ have the same charge but Z* is somewhat greater for Ca2+. Thus, Ca2+ will more effectively withdraw charge from the OH bond in coordinated water, leading to a slightly higher acidity and a slightly lower pH.
(c) 0.1 M Hg(NO3)2 or 0.1 M Zn(NO3)2
Hg2+ and Zn2+ have the same charge but Z* is much greater for Hg2+ because of poor shielding by the 4f electrons. Thus, Hg2+ will more effectively withdraw charge from the OH bond in coordinated water, leading to a higher acidity and a lower pH.
7. Draw the structure of chloric and chlorous acid and predict their pKa values using Pauling's rules.
Chloric acid is HClO3 and chlorous acid is HClO2:
Using Pauling's rules, HClO3 = (HO)ClO2 so pKa ~ 8 – 5(2) = –2 (a strong acid) and HClO2 = (HO)ClO so pKa ~ 8 – 5(1) = 3 (observed = 1.94 at 25 °C).
8. In the gas phase, the base strength of amines increases regularly along the series NH3 < CH3NH2 < (CH3)2NH < (CH3)3N. Consider the role of the steric effects and the electron-donating ability of CH3 in determining this order. In aqueous solution, the order is reversed. What solvation effect is likely to be responsible?
The acid-base reaction in the gas phase is:
B(g) + H+(g) → HB+(g)
Steric effects have a small role in this reaction since the gas phase hydrogen ion is so small. Thus, the electron donating ability of the methyl groups primarily influences the base strength: increasing the number of methyl groups increases the electron density in the nitrogen lone pair, leading to the increased base strength.
In aqueous solution, the trend mostly reverses (despite the statement in the problem, ammonia is not the strongest aqueous base in this series; the order in water is NH3 < (CH3)3N < CH3NH2 < (CH3)2NH). In water the base strength is determined by the reaction:
B(aq) + H2O(aq) → HB+(aq) + OH–(aq)
The bulky methyl groups prevent access of the water to the nitrogen lone pair (i.e., there is less hydrogen bonding) so the base strength results from a balance between steric effects and electron-donating effects.
9. The aqueous pKa values for HOCN, H2NCN, and CH3CN are approximately 4, 10.5, and 20 (estimated), respectively. Explain the trend in these –CN derivatives of binary acids and compare them with H2O, NH3, and CH4. Is –CN electron donating or withdrawing?
10. The hydroxoacid Si(OH)4 is weaker than H2CO3. Write balanced equations to show how dissolving a solid M2SiO4 can lead to a reduction in the pressure of CO2 over an aqueous solution. Explain why silicates in ocean sediments might limit the increase of CO2 in the atmosphere.
As the central atom in each of these species becomes less electronegative, the acidity decreases because the electronegative atom withdraws electron density from the acidic hydrogen atom. The order of increasing electronegativity is O > N > C so that HOCN (pKa ~ 4) is more acidic than H2NCN (pKa ~ 10.5), which is more acidic than CH3CN (pKa ~ 20). Likewise, water is more acidic than ammonia, which is more acidic than methane. Substituting –H by –CN increases the acidity in every case (for example, for HOCN pKa ~ 4 while for HOH pKa ~ 15) because the –CN group is strongly electron withdrawing. The cyano group withdraws electron density from the H-X bond, weakening the bond and increasing the acidity.
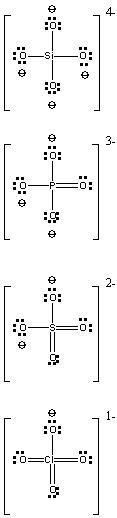
11. Draw the structures and indicate the charges of the tetraoxoanions of X = Si, P, S, and Cl. Summarize and account for the trends in the pKa values of their conjugate acids.
The Lewis structures of each of the ions is shown below. All of the ions are tetrahedral.
The conjugate acids are H4SiO4, H3PO4, H2SO4, and HClO4. The pKa1 values estimated by Pauling's rules are 8, 3, –2, and –7, respectively. Thus, the acidity increases moving across to the right on the Periodic Table, following the trend in Z*. As Z* increases, the central atom draws more electron density to itself from the OH bond, thereby weakening the bond.
12. An electrically conducting solution is produced when AlCl3 is dissolved in the basic polar solvent CH3CN. Give formulas for the most probable conducting species and describe their formation using Lewis acid-base concepts.
AlCl3 is a good Lewis acid but also can lose a Cl– fairly readily. Thus, two possible reactions are:
2 AlCl3 + CH3CN → AlCl4– + [AlCl2–NCCH3]+
2 AlCl3 + 2 CH3CN → AlCl4– + [AlCl2(–NCCH3)2]+
The first reaction is composed of two complex ion formation reactions. One reaction forms the tetrachloroaluminate anion and leaves the dichloroaluminum(III) cation, which then adds a solvent molecule to form the product shown.
The second reaction is the first reaction with one more complex formation, by adding the second solvent molecule to the cation. This might be favored since Al3+ has a tendency to be 4-coordinate.
13. Order the following cations in terms of increasing Brønsted acidity in water: Sr2+, Ba2+, Hg2+.
Z* will be highest for Hg2+ because of the presence of poor shielding d electrons, so this will be the strongest acid. Ba2+ is a bit larger than Sr2+ so will have a lower charge density. Thus, Ba2+ will be the weakest acid. The net order, then, is Ba2+ < Sr2+ < Hg2+.
14. Consider the three manganese oxides MnO, MnO2, and Mn2O7. One of them is acidic, one of them is basic, and one is amphoteric. Which one is which?
Acidic oxides arise from covalently bound O atoms. In transition metal compounds a high covalency is found in species with a higher formal oxidation state. For these three examples, Mn2O7 has manganese in the +7 oxidation state, so this is the acidic oxide. In contrast, basic oxides arise from ionically bound oxide. Here, MnO will be the most ionic so must be the basic oxide. MnO2 has Mn in the +4 oxidation state, an intermediate value, so is the amphoteric example.
15. For the reactions given below, find ΔH in units of kJ/mol.
C5H5N + SbCl5 → C5H5N–SbCl5
(CH3)2SO + SbCl5 → (CH3)2SO–SbCl5
Use the Drago-Wayland parameters:
EA(SbCl5) = 15.1
CA(SbCl5) = 10.5
EB(C5H5N) = 2.39
CB(C5H5N) = 13.10
EB((CH3)2SO) = 2.76
CB((CH3)2SO) = 5.83
ΔH(pyridine reaction) = –[(15.1)(2.39) + (10.5)(13.10)] = –173.6 kJ/mol
ΔH(DMSO reaction) = –[(15.1)(2.76) + (10.5)(5.83)] = –102.9 kJ/mol
16. Predict the products and write balanced equations for the following chemical reactions:
a. CH3HgI + HCl
b. SO3 + H2O
c. SO3 + NH3
a. CH3HgI + HCl
CH3HgI + HCl →← NR
CH3Hg+ is softer than H+ and I– is softer than Cl–. The soft acid is matched with the soft base and the hard acid is matched with the hard base. Thus, no appreciable reaction will occur.
b. SO3 + H2O
SO3 + H2O →← H2SO4
This reaction mixes Brønsted-Lowry and Lewis behavior. A hydrogen ion transfers to an oxygen atom on the sulfur trioxide to form HSO3+ and hydroxide ion. Then the hydroxide ion complexes to the HSO3+ ion to form the product.
c. SO3 + NH3
SO3 + NH3 →← H3N–SO3
This is a classic Lewis acid-base complex formation reaction.
17. Calculate the enthalpy of reaction for SbCl5 reacting with acetone and dimethylsulfoxide (two common solvents). Which solvent would have the higher donor number?
Use the Drago-Wayland equation to find the enthalpies of reaction.
For acetone:
SbCl5 + CH3COCH3 → ← SbCl5–CH3COCH3
–ΔH° = EACA + EBCB
–ΔH° = (15.1)(2.02) + (10.5)(4.67) = 79.5 kJ/mol
For DMSO:
SbCl5 + CH3SOCH3 → ← SbCl5–CH3SOCH3
–ΔH° = EACA + EBCB
–ΔH° = (15.1)(2.76) + (10.5)(5.83) = 102.9 kJ/mol
Since reaction with DMSO is more exothermic, it will have a higher donor number.
18. Would you expect Pauling's rules for calculating pKa to work for carboxylic acids? Why or why not?
The general formula for a carboxylic acid is RCO2H. In terms of Pauling's rules, there is 1 OH, 1 O, and a third group, R. The structural and electronic effects of the R group would be expected to negate the effectiveness of Pauling's rules. For example, for acetic acid, CH3CO2H, the predicted pKa is ~ 8 – 5 = 3, while the observed pKa = 4.74.
19. a) Amongst BF3, BCl3, and BBr3, which would be the strongest Lewis acid? Why? b) Which is more basic towards B(CH3)3: Me3N or Et3N? Why?
a) BBr3 is expected to be the strongest Lewis acid. This is because the Br is the least electronegative substituent atom. For more electronegative atoms, the electron density is pulled away from the central B atom, making it more positive, and shrinking the size of the empty p orbital, making it less available for accepting an electron pair from a Lewis base.
b) Me3N is expected to be a better Lewis base towards B(CH3)3 for steric reasons. Both bases are bulky, but Et groups are larger, hence will flatten out Et3N, and will prevent a close approach from the Lewis acid.