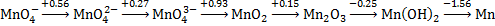Chemistry 401
Intermediate Inorganic Chemistry
University of Rhode Island
Homework 3
1. (10 pts) The Latimer Diagram for manganese in base is shown below. Using this, answer the following questions
a. Write the balanced half-reaction for the reduction of permanganate ion to dimanganese trioxide solid.
b. Find the standard reduction potential for the reaction in part a.
c. Are any of the manganese species in aqueous base subject to disproportionation? If yes, write the reaction. If no, explain why not.
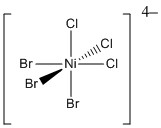
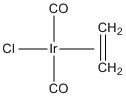
2. (40 pts) For the following species, find the LFSE (in units of Dq and P), give the point group, give the systematic name, predict the spin-only magnetic moment in units of Bohr-magnetons, and indicate if the species is Jahn-Teller active.
a. [Co(NH3)6]3+
b. [Cr(CN)6]3–
c.
d.