Chemistry 401
Intermediate Inorganic Chemistry
University of Rhode Island
Homework 1
1. Write Lewis structures for the molecules or ions shown below. Show all resonance structures, including those that follow the octet rule (if possible) and the minimum formal charge rule. Do not include structures that are ionic. Indicate which structure(s) will be the lowest energy. Explain your reasoning.
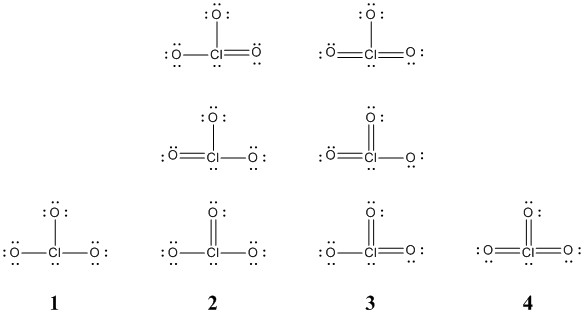
a. ClO3– (eight structures, show four of different energies)
The eight resonance structures for chlorate ion are shown below:
Structure 1 has octets on all atoms and the formal charges are –1 on all O atoms but +2 on the Cl atom. Consequently, this is unlikely to be the lowest energy structure.
Structures 2, 3, and 4 keep octets on the O atoms but Cl atom does not meet the octet rule. In these structures the charge on the Cl atom is +1, 0, and –1, respectively. Structure 3 is probably lowest in energy since O and Cl have similar electronegativities. However, structures 1 and 4 are certainly higher in energy.
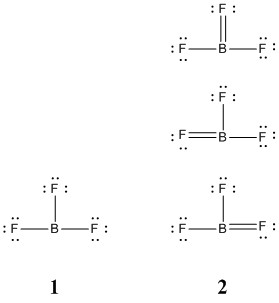
b. BF3 (four structures, show two of different energies)
The four resonance structures for boron trifluoride are shown below:
Structure 1 has minimum formal charges (all atoms have a formal charge of 0) while structure 2 meets the octet rule for all atoms. Structure 1 is energetically preferred because of the 0 formal charge on all atoms. While structure 2 has octets, the formal charge on the F atoms is positive, +1/3, which is inconsistent with the electronegativities of B and F.
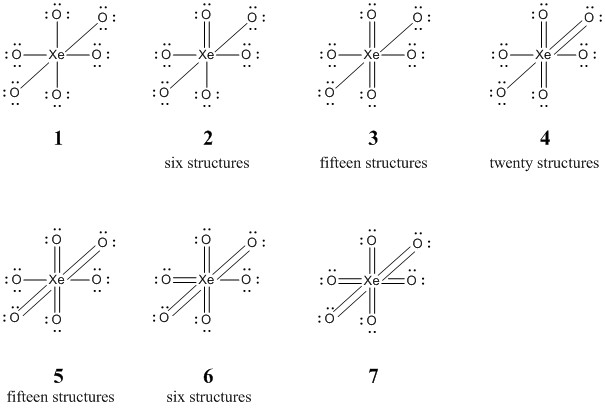
c. XeO64– (sixty-four structures, show seven of different energies)
Seven of the resonance structures for perxenate ion are shown below:
There are no structures with an octet on the Xe atom.
Structures 1 and 2 have a positive formal charge on the Xe, structure 3 has 0 formal charge on the Xe, and structures 4, 5, 6, and 7 have negative formal charge on the Xe. Structure 3 is probably the lowest energy but structure 2 is likely close. However, structures 4, 5, 6, and 7 are certainly higher in energy.
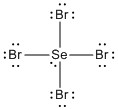
d. SeBr4 (you decide)
There is only one reasonable Lewis structure:
There is no structure with an octet on the Se atom.