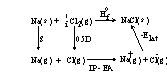Chemistry 401
Measuring Lattice Energies
Direct measurement is possible but experimentally difficult and low accuracy:
M+(g) + X–(g)→ ← MX(s) ΔHo
Better to use a thermodynamic cycle; a Born-Haber cycle:
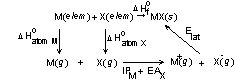
ΔHoatom M + ΔHoatom X + IPM – EAX – Elat – ΔHfo = 0
solve for Elat by measuring all the other parameters
NaCl:
S + 0.5D + IP – EA – Elat – ΔHfo = 0 so Elat = S + 0.5D + IP – EA – ΔHfo
Elat = (+108.4) + 0.5(+241.8) + (+495.4) - (+348.5) - (-410.9) = 787.1 kJ/mol
What do we mean by ionic size?
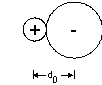
We can only measure the distance between ions experimentally; however, this distance should equal the sum of the cation size and the anion size, so
do = r+ + r–
r+ = cation radius, r– = anion radius
How is the sum apportioned?
Landé used LiI as the starting point: rock salt lattice. Iodides form lattice and are "touching" because of their large size and the small size of Li+ which fits completely in the Oh holes (an assumption). For a lattice constant = a, then √2a = 4r– and a = 2r+ + 2r– so that each ionic radius can be found.
Now do the same experiment for other ionic salts without making the "touching" assumption. A whole set of self-consistent ionic radii can then be found.
Results are reasonable but not perfect. Ionic radii depend on coordination number: ions in Td holes have different radii than ions in Oh holes. When using ionic radii, always need to use a self-consistent set of values and to know the coordination number.