Chemistry 401
The Solid State
In solids, atoms or molecules are near each other to the point of 'touching' (high density)
In the simplest case, a solid can be thought of as an array of spheres put together in some arrangement (spherical symmetry certainly is a good assumption for metals, perhaps poorer for molecular solids)
The packing of spheres can fit into one of two extremes:
Crystalline Solids : x-rays diffract sharply which implies regular order on the scale of nm
spheres pack into a regular, repeatable fashion called the crystal lattice
the smallest repeat unit in the lattice is called the unit cell
the unit cells fit together snugly like bricks in a wall-this is translational symmetry
Amorphous Solids: x-rays scatter broadly and diffusively which implies no long range order
spheres pack together closely but there is no translational symmetry
no regular order to the unit cells (there still may be short range order)
like the pile of bricks before being laid into a wall
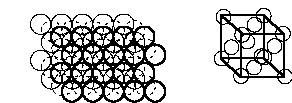
Closest packed structures
ABAB (hexagonally closest packed, hcp)
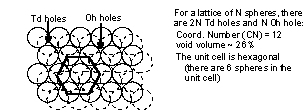
Coordination Number = CN = the number of nearest neighbors
Td = tetrahedral holes (4 nearest neighbors, CN = 4)
Oh = octahedral holes (6 nearest neighbors, CN = 6)
ABCABC (cubic closest packed, ccp or face centered cubic, fcc)
fcc still has CN = 12, still 26% void volume, the unit cell has 4 spheres in it.
Closest packed structures are the most efficient way to pack spheres but are not required or even the most common.
Other common structures:
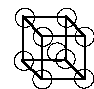
body centered cubic (bcc):
spheres only 'touch' along the body diagonal of the cubic unit cell
CN = 8, 2 spheres per unit cell, ~32% void volume
primitive cubic:
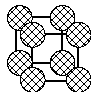
spheres only touch along cell edges, CN = 6, void volume ~48%
1 sphere per unit cell
The structure of metals often changes as a function of temperature - phase transitions occur - this is called polymorphism or polytypism
Alloys are form by mixing different types of metals together:
Substitutional alloys: one metal replaces another metal at some lattice sites. This requires that both types of metals be roughly the same size.
Interstitial alloys: the new atoms are introduced into the hole sites (called interstices) of the host lattice. The new atoms can occupy either Td or Oh holes.
Ionic solids
Ionic materials form crystalline lattices like metals but the lattice structure is determined by one ion (the larger, usually the anion) and the other ion fits into the holes. Since the smaller ion may not just right, lattice expansions can occur.
What holds an ionic lattice together?
A simple model: assume each ion is represented by a point charge, then sum up all the Coulomb interactions.
ECoul = NAAq+q– 4πεod
q+, q– are charges on the ion, d the distance between ions, ε0 = permittivity of free space, NA is Avogadro's number, A is the Madelung constant
The Madelung constant is a purely geometric factor that has been tabulated for many lattice types. It represents the potential energy associated with different relative geometries. d is the distance between ions so accounts for the different energy associated with different compounds with the same lattice type (hence, the same Madelung constant).
ECoul is negative, thus attractive, but maximizes at d = 0, i.e. when anions and cation occupy the the same point in space. Need a repulsive term (to account for nuclear-nuclear repulsion when the ions are close enough) which is done in an ad hoc fashion.
Born-Mayer: Erep = Ced*/d where C and d* are constants
Born-Landé: Erep = B/dn where B and n are constants
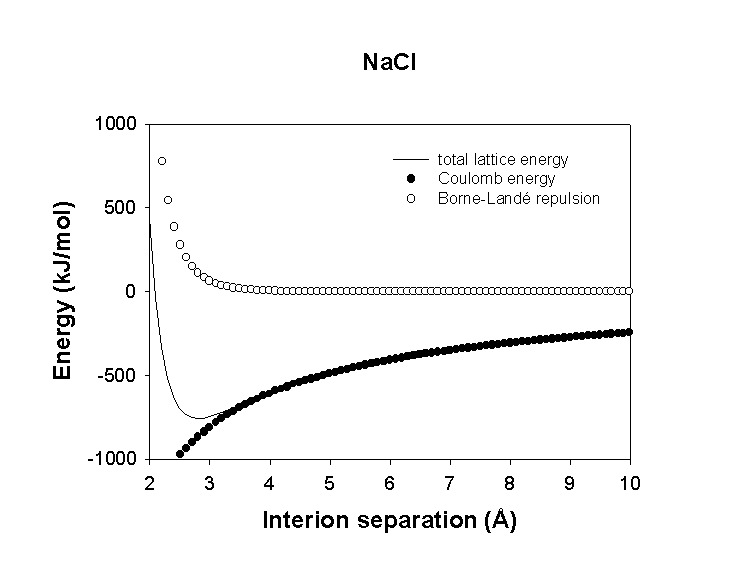
Then, Elat = ECoul + Erep graphically:
The total lattice energy has a minimum which is the equilibrium value for d (i.e., the observed distance between ions in a lattice). Erep is larger than ECoul at small distances (for large n or d*) but the reverse is true at short distances. We evaluate the constants by using experimental data at equilibrium and compressibility (this gives the slope up the low d part of the graph)
Elat = NAAe2Z+Z– 4πεodo (1 – 1/n) Born-Landé
Elat = NAAe2Z+Z– 4πεodo (1 – d*/do) Born-Mayer
e = electronic charge, Z = ion charge in units of e (i.e., +1, +2, –1, –2, etc.)
Note: the lattice energy is only a slight modification of a simple dipole energy! (n ~ 5-10)
For NaCl
do = 2.814 Å = 2.814×10–10 m
A = 1.748
n = 7.66
NA = 6.022×1023 mole–1
e = 1.602×10–19 C
4πεo = 1.113×10–10 J–1 C2 m–1
Elat = (6.022×1023 mole–1) (1.748)(1.602×10–19 C)2(+1)(–1) (1.113×10–10 J–1 C2 m–1) (2.814×10–10 m)(1 – 1/(7.66)) = –750.0 kJ/mol
experimental value = 787 kJ/mol
note the sign difference: Elat is usually written without the negative sign (thermodynamically wrong)