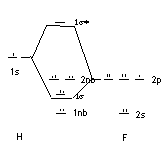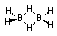Chemistry 401
MO Diagrams for Heteronuclear Diatomic Molecules
HF
Basis functions: H 1s; F 2s, 2p
Energy of basis functions : 1s Z = 1, F, Z* >1 therefore 2s, 2p lower in energy
BO = 1 σ bond more centered on F so electron density is favors this atom
This is consistent with our ideas about electronegativity
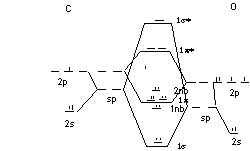
CO
basis, 2s, 2p on each atom; O orbitals a little bit lower
BO = 3 lone pair on C accounts for Lewis basicity
2nb:
1π*
1π
MO diagrams for Polyatomic molecules
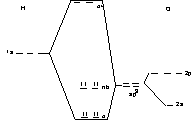
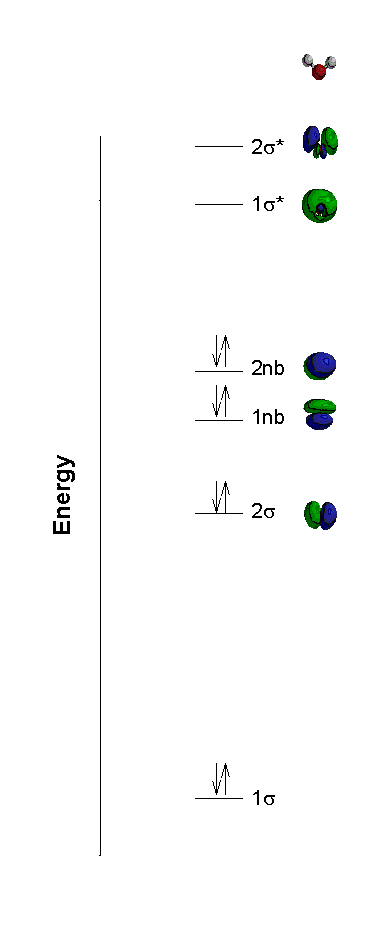
H2O
BO = 2
Problem : experimentally, the σ orbitals are of different energy, as are the nb orbitals.
This problem can not be fixed predictably with the methods we have available
Using more sophisticated MO calculations gives:
B2H6 VBT Lewis Dot structure doesn't have enough electrons:
The B-H-B bonds are called electron deficient bonds (3-center, 2-electron bonds)
MO theory ; break it up into BH3 fragments