Chemistry 401
Molecular Orbital Theory
Experimentally, O2 is found to have two unpaired spins – VBT can not explain a simple molecule like O2 !
Linear Combination of Atomic Orbitals
Assumptions:
1) Molecular Orbitals look like atomic orbitals, especially near the nucleus
2) Molecular Orbitals are constructed by interference patterns of atomic orbitals
3) The total number of molecular orbitals = the total number of atomic orbitals
4) Molecular wavefunctions are products of the molecular orbital functions
Overlap of atomic orbitals
constructive interference gives bonding orbital, no nodes
destructive interference gives antibonding orbital one node, perpendicular to the internuclear axis
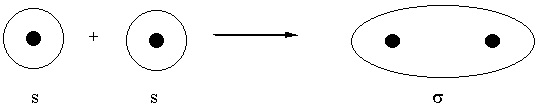
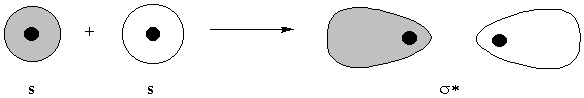

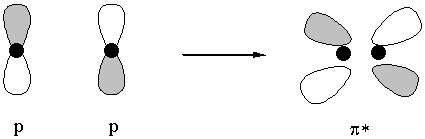
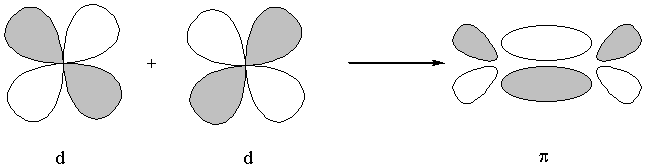
molecular orbitals are labeled by nodal properties:
0 nodes parallel to the internuclear axis: σ
1 node parallel to the internuclear axis: π
2 nodes parallel to the internuclear axis: δ
0 nodes perpendicular to the internuclear axis: bonding
1 node perpendicular to the internuclear axis: antibonding
Construction of Energy Level diagrams
1) Define the basis set (i.e., the set of atomic orbitals to be used, generally the valence orbitals)
2) # of MOs = # of AOs
3) The change in energy is proportional to the degree of overlap
4) Better overlap occurs between AOs of about the same energy
5) Bonding orbitals lower energy, antibonding orbitals raise energy relative to the AOs
Bond Order = BO = ½(# electrons in bonding orbitals - # electrons in antibonding orbitals)
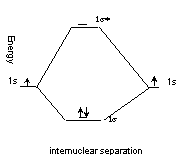
H2
BO = 1, same as VBT
First row diatomics basis set : 2s and 2p (1s is a core orbital, so will not be affected much by bonding)
A2configurationB.O. predicted spinsobserved spins
Li21σ21 00
Be21σ21σ*2 000
B21σ21σ*21π2 122
C21σ21σ*21π4 200
N21σ21σ*22σ21π4 300
O21σ21σ*22σ21π41π*2 222
F21σ21σ*22σ21π41π*4 100
Ne2 1σ21σ*22σ21π41π*42σ*2 000