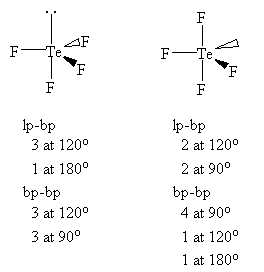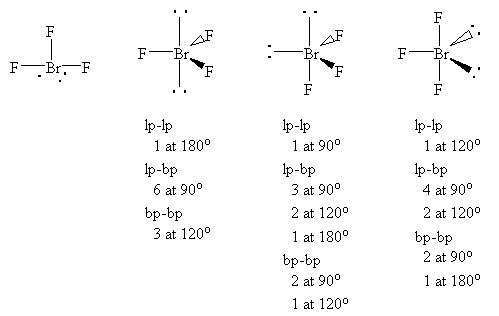Chemistry 401
VSEPR : Valence Shell Electron Pair Repulsion model
VSEPR uses Lewis dot structures and a simple electrostatic model to predict molecular geometries
1. Write the Lewis dot structure with the lowest possible formal charges.
2. Determine the total number of bonds+lone pairs around the center of interest.
(note : sb = db = tb in this count)
3. Distribute these electrons according to the following scheme (based on minimizing repulsion):
countdistribution geometryshape
2linear
3trigonal
4tetrahedral
5trigonal bipyramidal
6octahedral
4. Add in atoms and assign lone pairs to minimize total repulsions
lp-lp > lp-bp > bp-bp (tb > db > sb)
Structure Prediction using VSEPR
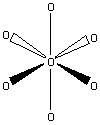
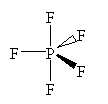
PF5
TeF4
BrF3