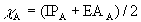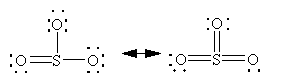Chemistry 401
Electronegativity
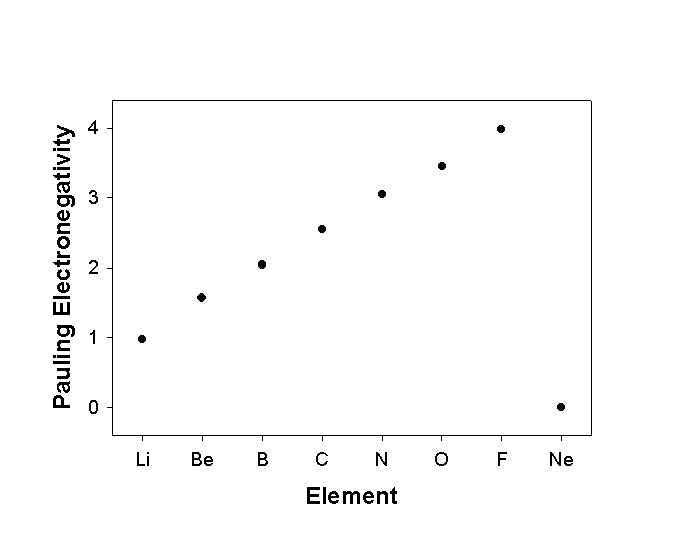
Pauling defined electronegativity as "the power of an atom to attract electrons to itself in a bond"
Pauling Scale:
where:
Δ = E(A-B) – [E(A-A) + E(B-B)]/2
E(A-A), E(B-B), E(A-B) are bond energies in kJ/mol
Allred-Rochow scale: suggested that electronegativity is an electrical force
where:
rcov is covalent radius in Angstroms
Mulliken: made electronegativity into a true atomic property; now called absolute EN
Covalent Bonding
Bonding between atoms occurs because electron pairs are shared between atoms.
Why does this cause a nuclear-nuclear attraction?
Accumulated electron density causes a net nuclear-nuclear attraction (increased electron-nuclear attraction is greater than the increased electron-electron repulsion)
Valence Bond Theory: at an elementary level, electrons occupy a volume of space and orbitals are constructed to match the electron occupation.
Molecular Orbital Theory: orbitals are constructed and then filled with electrons according to the Aufbau and Pauli Principles.
Lewis Dot Structures: the usual method of denoting VBT bonding
Classical description/rationalization: atoms in molecules strive to attain a rare gas electron configuration
This is probably a bad generalization for most of the Periodic Table
two electrons on each atom
eight electrons on each atom
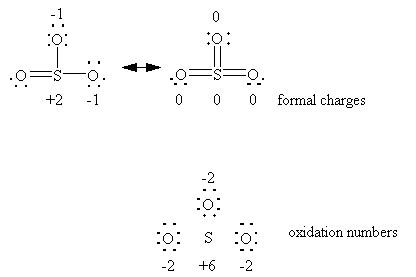
Better: atoms share electrons in molecules to attain the minimum formal charges
Formal charge: found by splitting the electrons in each equally between the bonded atoms.
Oxidation number: found by assigning all the electrons in a bond to the more electronegative atom.
Reality : somewhere in between