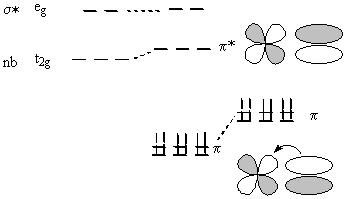Chemistry 401
Molecular orbital approach (Ligand Field Theory)
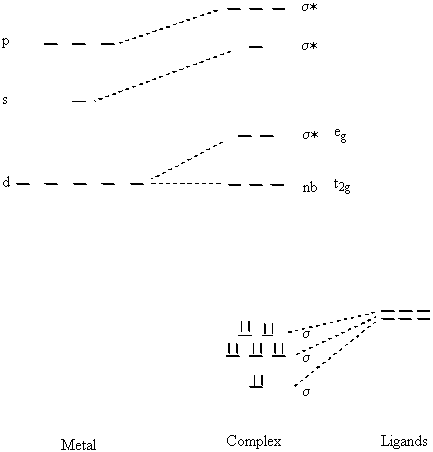
A Molecular Orbital Diagram using only sigma bonding can be constructed for a general Oh complex:
Bond Order = 6 - n(eg)/2
The sigma bonds are largely ligand based orbitals but take the appearance of d2sp3 hybrid orbitals about the metal.
The nb and lowest energy σ* orbitals are similar to the Crystal Field Theory description. The nb orbitals are strictly d like (dxy, dyz, dxz) and the σ* are mostly d orbital like (dx2–y2, dz2).
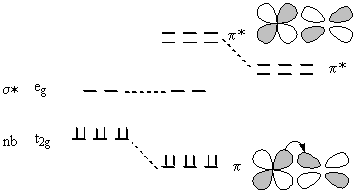
Inclusion of π bonding means using the t2g orbitals for overlap:
If the t2g orbitals are empty, then the ligand needs filled π orbitals to create a M-L π bond; the t2g orbitals become somewhat antibonding, are destabilized, and 10Dq is reduced.
If the t2g orbitals are occupied, then the ligand needs unfilled π* orbitals to create the M-L π bond; the t2g orbitals become somewhat bonding, are stabilized, and 10 Dq is increased.
This is known as π backbonding. It is the reason CO is such a strong ligand.