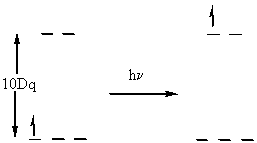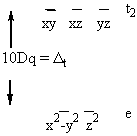Chemistry 401
Measurement of 10Dq
Usually done spectroscopically, move electron from t2g to eg orbital with no spin change
hν = 10Dq in this case
Because of electron–electron repulsion, the lowest energy transition is not always equal to 10Dq.
ConfigurationLowest Energy Spin–Allowed Transition
d110Dq
d28Dq
d310Dq
d4 (hs)10Dq
d4 (ls)~ 9Dq
d5 (hs)none
d5 (ls)~ 8.5Dq
d6 (hs)10Dq
d6 (ls)~ 9Dq
d7 (hs)10Dq
d7 (ls)~ 9Dq
d88Dq
d910Dq
d10none
Complication : charge transfer transitions
M–L →hν M+–L– Metal to Ligand Charge Transfer (MLCT)
M–L →hν M––L+ Ligand to Metal Charge Transfer (LMCT)
CT transitions are usually much more intense than d–d transitions so can be distinguished by molar absorptivity
ε (CT) ~ 103 – 104 L/mol–cm
ε (d–d spin allowed) ~ 101 – 102 L/mol–cm
ε (d–d spin forbidden) ~ 10–1 – 100 L/mol–cm
How does Crystal Field theory change if the complexes are not octahedral?
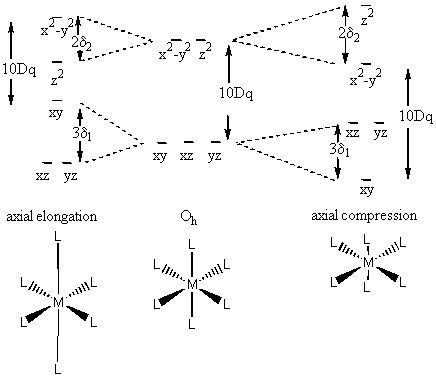
Consider a tetragonal case:
Need to introduce additional parameters, δ1 and δ2
The situation is such that E(x2–y2) – E(xy) = 10Dq (moving the ligand along the z axis should have no effect on the relative energies of the orbitals in the xy plane).
For compression case :
[E(x2–y2) + δ2] – [E(xy) + 2δ1] = 10Dq
[E(x2–y2) – E(xy)] + δ2 - 2δ1 = 10Dq
10Dq + δ2 -2δ1 = 10 Dq
δ2 = 2δ1
Can we predict when this will happen? Yes, using the Jahn-Teller theorem
Jahn-Teller Theorem: In a nonlinear molecule a degenerate electronic state will distort to remove the degeneracy and to increase the stability
Consider d1
In an Oh geometry, the electronic state is triply degenerate (the single electron can be in one of three orbitals of identical energy).
Axial elongation gives a state that is still degenerate (doubly) so would need to further distort.
Axial compression leads to a singly degenerate state and increased stability.
LFSE = –4Dq – 2δ1
This should occur even if all the ligands are the same!
Which configurations should be J-T active?
configurationJ-T active?distortion
d1yescompression
d2yeselongation
d3no
d4 (hs)yeseither
d4 (ls)yescompression
d5 (hs)no
d5 (ls)yeselongation
d6 (hs)yescompression
d6 (ls)no
d7 (hs)yeselongation
d7 (ls)yeseither
d8no
d9yeseither
d10no
Tetrahedral Complexes
Tetrahedral symmetry is fairly common but can not be treated as a distortion from Oh
Ligands between axes are destabilized, ligands along axes are stabilized.
The splitting in Td complexes is always less than the splitting in Oh complexes with the same ligands (Δt < Δo). (Fewer ligands give a smaller electrostatic field; in the exact ionic limit Δt = 4 9Δo.)
This means that Td complexes are always high spin and usually bluer.