
Chemistry 401
Crystal Field Theory or Ligand Field Theory
An ionic approach to understanding bonding in transition metal complexes
Consider the Oh case : what is the effect of the negative electron density from the ligands on the energies of the valence (d) orbitals
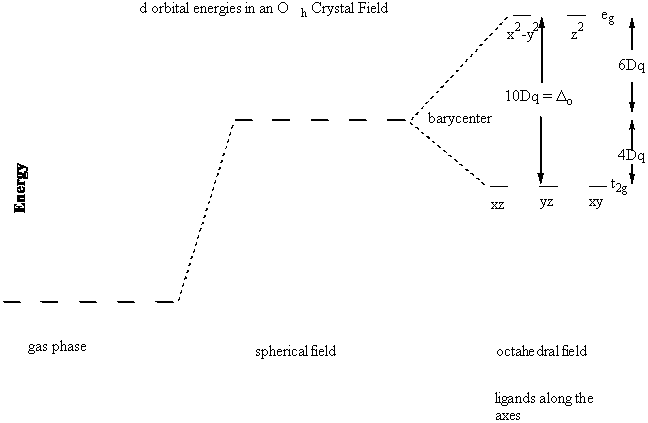
Δo = 10Dq = Crystal Field Splitting parameter or Ligand Field parameter
Occupation of the t2g orbitals gives a little extra stabilization of the complex
Ligand Field Stabilization Energy (LFSE)
d electron configurationLigand Field configurationLFSE unpaired electrons
d1t2g14Dq 1
d2t2g28Dq 2
d3t2g312Dq 3
d4t2g416Dq – P 2 (ls)
d4t2g3eg16Dq 4 (hs)
d5t2g520Dq – 2P 1 (ls)
d5t2g3eg20Dq 5 (hs)
d6t2g624Dq – 2P 0 (ls)
d6t2g4eg24Dq 4 (hs)
d7t2g6eg118Dq – P 1 (ls)
d7t2g5eg28Dq 3 (hs)
d8t2g6eg212Dq 2
d9t2g6eg36Dq 1
d10t2g6eg40Dq 0
P = spin pairing energy
hs = high spin
ls = low spin
Influences on Dq:
metal : charge, size (Z*)
ligands : charge, orbitals available for bonding (σ, π), ring formation
Spectrochemical series
ligands ordered by relative size of Dq for any metal ion ligands ordered by ligand field strength
I– < Br– < S2– < SCN– < Cl– < NO3– < F – < ox2– < H2O < SCN– < CH3CN < NH3 < en < bipy < phen < NO2– < PPh3 < CN– < CO
High spin vs low spin
Complexes are high spin if Dq is small : weak field case
Complexes are low spin if Dq is large : strong field case
Magnetic susceptibility measurements, which measure the size of the magnetic moment, are used to distinguish the spin state.
The magnetic moment reflects the total available angular momentum in transition metal complexes (especially first row), most of this comes from spin (the orbital contribution is said to be quenched):
μ = g[S(S+1)]½μB
g ~ 2 (fundamental constant); μB = Bohr magneton
Since each unpaired electron has S = ½
μ = [n(n+2)] ½ μB
n = number of unpaired spins