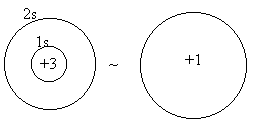Chemistry 401
Multielectron atoms
We can not solve the Schroedinger equation exactly so assumptions must be made.
Usual assumption: the hydrogenic obitals are adequate and electrons occupy them in some fashion.
The occupation of electrons in orbitals is called the electron configuration – this is one way to describe the electronic properties of atoms.
Two guiding principals used to account for electron configurations:
Aufbau Principle: electrons occupy orbitals in such a manner to give the lowest possible total energy
Pauli Exclusion Principle: each electron in an atom is described by a unique set of quantum numbers (n, l, ml, ms) - i.e., two electrons per hydrogenic orbital
Periodic Table: based on electron configurations and can be used to predict them but not absolute (electron configurations are experimental quantities)
Aids in finding correct electron configurations from the Periodic Table:
Half-filled phenomenon: when d or f electrons are the valence shell, if a shift of 1 electron (occasionally 2 but this is not predictive) from an s orbital to the d or f orbital leads to a filled or half filled d or f orbital, this will stabilize the electron configuration.
Anions: add electrons to the neutral atom and follow above rules.
Cations: electrons are always removed from the orbitals of neutral atoms with the largest principal quantum number (n); the remaining electrons fill the orbitals with the lowest n consistent with the Pauli Principle
Term Symbols
Another way to indicate electron configurations
A term symbol gives the required information about the angular momentum quantum numbers l, m<l, and ms
For Ground States of atoms:
1) Only consider unfilled subshells; filled shells do not contribute
2) Fill the valence electrons into the unfilled subshell such that the highest total spin is attained using the highest possible ml values (this is Hund's Rule)
3) L = Σml
4) S = Σms
5) The term symbol is written as 2S+1L
2S+1 is called the total spin multiplicity or degeneracy and is written as a number
L is the total orbital angular momentum and is written as a letter:
Lletter designation
0S
1P
2D
3F
4G
5H
6I
Periodic Properties
Consider Li:
How good is such an approximation?
pretty good for s orbitals - they overlap the nucleus
mediocre for p orbitals - nodal at the nucleus
poor for d or f orbitals - multiply nodal at the nucleus
The reduction of nuclear charge seen by outer electrons due to the inner electrons is called screening or shielding
quantitatively:
Z* = Z - σ
Z* = effective nuclear charge
Z = true nuclear charge
σ = shielding constant
Shielding constants are established by ad hoc rules or quantum mechanical calculations
Trends in Z*
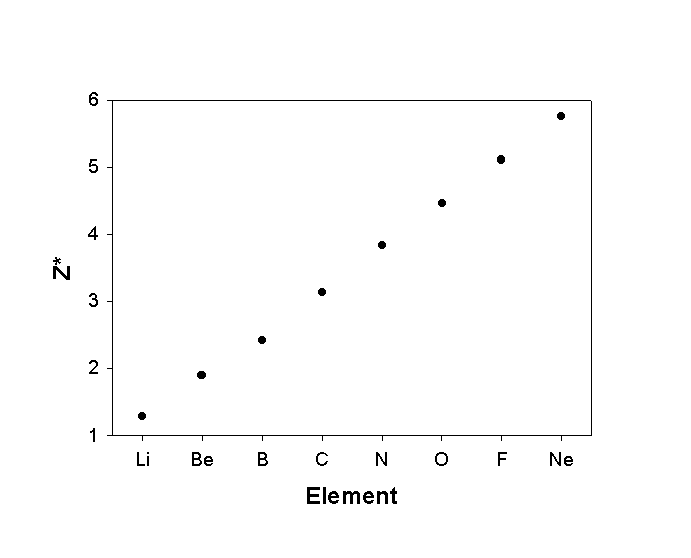
s orbitals are good screeners, p orbitals are moderate screeners; d, f orbitals are very poor screeners.
This means changes across the Table are bigger than changes down the Table
Experimental Properties
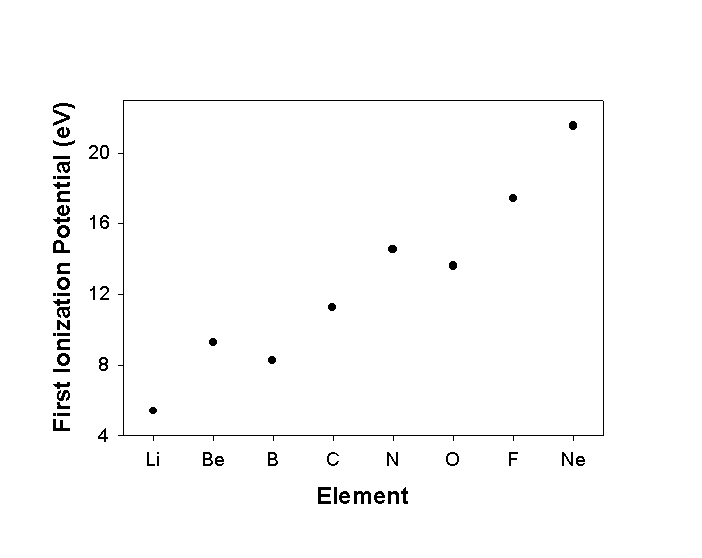
Ionization Potential (IP) or Ionization Energy (IE)
A(g)
A+(g) + e–
The energy associated with this reaction is the ionization energy
IP or IE is thermodynamically positive
IP generally follows Z* across the Table
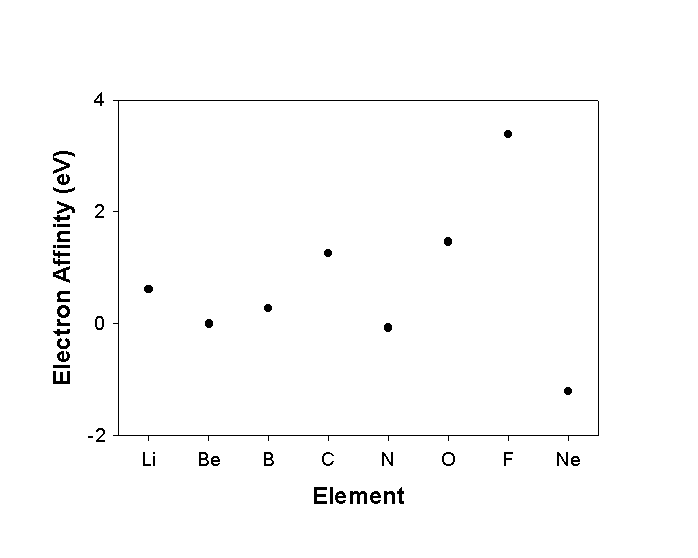
Electron Affinity : EA
A(g) + e–
A–(g)
EA reported with the wrong thermodynamic sign
Periodic trends are reverse of IP
Atomic Size : Radius of an atom - a difficult concept to define
atomic radius depends upon the bonding situation
metallic radius : ½ the internuclear distance between atoms in the metallic state
covalent radius : ½ the internuclear distance in a homonuclear covalent bond
ionic radius : size of an ion in a solid
van der Waals radius : the distance at which an atom just starts to respond to interactions from neighboring atoms
radii all of different numerical values but similar periodic trends:
r increases as go down the Periodic Table (bigger n)
r decreases as go across the Periodic Table (bigger Z*)