
Chemistry 401
Ligands
Usually ligands are Lewis bases (nonmetals, either anions or neutrals with lone pairs) but this is not required.
The ligand could be another metal complex to give a multimetal compound
Re2Cl82– (D4h)
(quadruple metal-metal bond)
Traditional ligands have N, O, halide, S, P as the binding site (all have lone pairs)
Binding of C to metals is also well known - this is organometallic chemistry
Ligands may have multiple binding sites:
monodentate : one binding site NH3, Cl–, H2O, CN–
ambidentate : two available binding sites but only one can be used CN–, SCN–
This gives the possibility of linkage isomerization : M-SCN differs from M-NCS
bidentate : two binding sites and both used
ethylenediamine (en), oxalate (ox), 2,2'-bipyridine (bipy), acetylacetonate (acac), phenanthrene (phen)
chelation : ligand plus metal form a ring system; requires a multidentate ligand
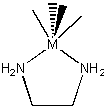
tridentate : three binding sites used
H2NCH2CH2NHCH2CH2NH2 (dien)
quadridentate : four binding sites
porphyrin
polydentate : many binding sites
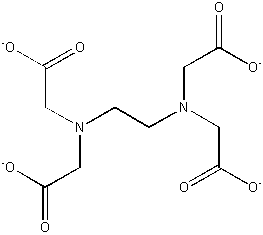
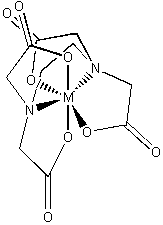
EDTA4– (–O2CCH2)2–NCH2CH2N– (CH2CO2–)2
binds strongly to nearly all metals; used to treat heavy metal poisoning
Nomenclature of transition metal complexes
Cationic and Neutral complexes:
1) ligands are listed in alphabetical order using systematic name except:
a) if the ligand name ends in -ide, -ate, or ite, drop the final e and and add an o
b) others : O2– oxo; OH– hydroxo; CN– cyano; O22– peroxo; NH3 ammine; H2O aqua; NO nitrosyl; CO carbonyl; F– fluoro; Cl– chloro; Br– bromo; I– iodo
2) the number of times a ligand appears is prefixed to the ligand using
StoichiometryPrefixAlternateuse the alternate when the preferred prefix gives ambiguity
2bibis
3tritris
4tetratetrakis
5pentapentakis
6hexahexakis
3) structural information prefixes ligands, and can be for each ligand if necessary
cis-; trans-; fac-; mer-; μ- (ligand bridges two metals);
ηx– (x = number of C bonded to metal in an organic ligand)
4) metal is last followed by parentheses with the formal oxidation state in roman numerals
5) if cationic, counterion is the second word
Examples
Co(NH3)6Cl3
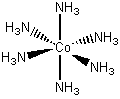
hexaamminecobalt(III) chloride
[Co(NH3)5Cl]Cl2
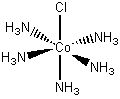
pentaamminechlorocobalt(III) chloride
[Co(NH3)4Cl2]Cl
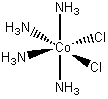
cis-tetraamminebichlorocobalt(III) chloride
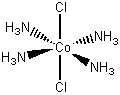
trans-tetraamminebichlorocobalt(III) chloride
[Co(NH3)3Cl3]
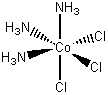
fac-triamminetrichlorocobalt(III)
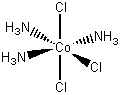
mer-triamminetrichlorocobalt(III)
CuCl2(CH3NH2)2
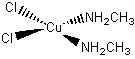
cis-bichlorobis(methylamine)copper(II)
Ni(CO)4
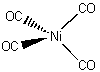
tetracarbonylnickel(0)
PtCl2(CH2=CH2)
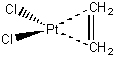
bichloro-η2-etheneplatinum(II)
(C5H5)2Fe
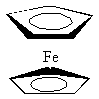
bis(η5-cyclopentadienyl)iron(II) [more commonly known as ferrocene]
Br2Pt-(S(CH3)2)2-PtBr2
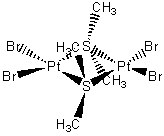
bis(μ-dimethylsulfido)bis(dibromoplatinum(II))
Anions:
1) counterion is first word
2) use cationic rules except add -ate to the end of the metal before the oxidation state info sometimes can use the Latin root
Examples
K2[OsCl5N]
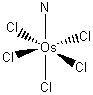
potassium pentachloronitridoosmate(VI)
Na3[Ag(S2O3)2]
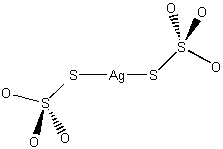
sodium bis(thiosulfato)argentate(I)
Na2[Fe(CN)5NO]
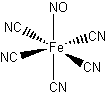
sodium pentacyanonitrosylferrate(III)