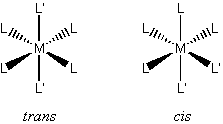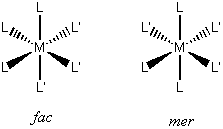Chemistry 401
Symmetry and Point Groups
Find the point group for the following species:
H2O
NH3
CCl4
[PtCl4]2– (square planar)
PF5
BrF3
XeO3
[Cr(NH3)6]3+ (ignore H atoms)
CH3CH3
Transition Metal Chemistry
d block chemistry is vast, often thought of in terms of Lewis Acid/Base interactions
Metals act as Lewis acids, which react with Lewis bases
The Lewis acid/base product is generally called a coordination complex and the Lewis bases are called ligands
Wide variety of d-block chemistry to be addressed:
geometries: coordination number (CN = the number of Lewis bases bonded to the metal) can vary from 2 to 8; 4 and 6 are most common
oxidation states: from negative to +7 or +8
bonding: nearly purely ionic to nearly purely covalent; intermediate much more common
reactivity: substitution, addition, elimination, redox - and a number of mechanisms
Structures of Coordination Complexes
CN = 2, 3
Rare, follow VSEPR rules CN =2, linear [Ag(NH3)2]+; CN = 3, trigonal planar
CN = 4
Quite common; found in two geometries-tetrahedral (Td) and square planar (D4h) some complexes are known that are between Td and D4h (D2d)
Square planar complexes can exhibit geometric isomerism: ML2L'2 cis or trans makes a difference in the chemistry: cis-Pt(NH3)2Cl2 is an anticancer drug, the trans isomer is inert
CN = 5
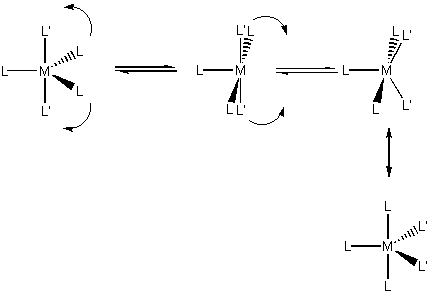
Not rare, not common; two geometries-trigonal bipyramid (D3h) and square-based pyramid (C4v); the two geometries are generally close in energy so ligands can scramble.
Berry pseudorotation is seen in mixed ligand complexes, this scrambles ligand positions:
CN = 6
Most common coordination number, octahedral
Often can get distortions from Oh
Extension or compression along one axis : tetragonal : D4h
Change along two axes : rhombic : D2h
Change along the diagonal (the C3 axis) : D3d
Substitutions:
disubstituted ML4L'2 : cis (C2v) or trans (D4h)
trisubstituted ML3L'3 : fac (C3v) or mer (C2v)
CN = 8
Very rare for first row (steric), found occasionally in 2nd or 3rd row complexes