Chemistry 401
Symmetry and Point Groups
Symmetry is one way to indicate geometrical aspects of a molecule (or object)
Symmetry elements are geometric operations that leave the appearance of a molecule identical (in all respects) to the starting point:
Types of symmetry elements
1. Inversion i(x,y,z) → (–x,–y,–z)
2. Rotations Cnrotates the molecule by 360°/n; by convention, rotations are clockwise; successive rotations are denoted by superscripts:
C62 = C3
C32 = 120° rotation counterclockwise
0° rotations are denoted by E.
Infinitely small rotations are denoted by C∞
3. Mirror planes σ reflection through a plane; the plane is often denoted by a subscript
a mirror is a composite symmetry element : σxy = C2zi
mirrors through bonds are usually denoted σv
mirrors between bonds are usually denoted σd
mirrors perpendicular to the highest n rotation axis are usually denoted σh
4. Improper rotations Sn a rotation followed by a mirror perpendicular to the rotation
Sn = Cnσh this can exist independent of the rotation or the mirror, e.g. S4 in CH4
note: S2 = σC2 = i
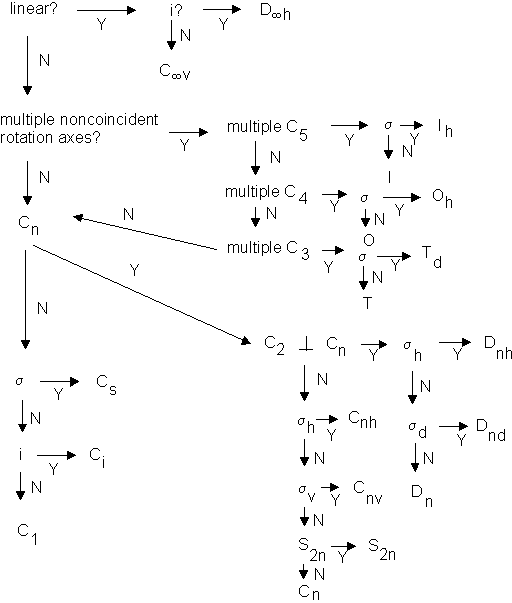
Point Group : the collection of all of the symmetry operations present in a molecule
Point Groups have mathematical significance, but we will take them simply as geometrical descriptions of molecules
To find point groups, we only need to identify certain key symmetry elements