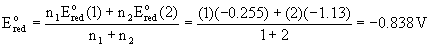Chemistry 401
Latimer Diagrams
This is a method of conveying large amounts of reduction potential information about a series of reductions for a given element.
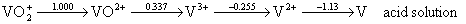
Simple redox reactions:
VO2+(aq) + 2 H+(aq) + e– → VO2+(aq) + H2O(l) Eored = +1.000 V
VO2+(aq) + 2 H+(aq) + e– → V3+(aq) + H2O(l) Eored = +0.337 V
V3+(aq) + e– → V2+(aq) Eored = –0.255 V
V2+(aq) + 2 e– → V(s) Eored = –1.13 V
More complex redox reactions (one of many possible examples)
V3+(aq) + 3 e– → V(s)
Disproportionation (one of many possible examples)
2 V3+(aq) + H2O(l) → V2+(aq) + VO2+(aq) + 2 H+(aq)
E°= –0.255 + –0.337 = –0.592 V
(disfavored, rather, the comproportionation reaction will occur)
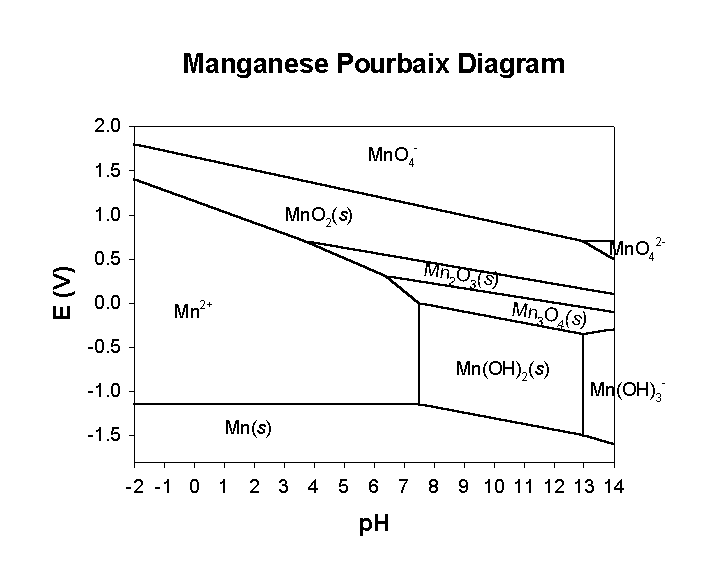
Pourbaix diagrams
These are phase diagrams for the stability of various species as a function of both pH and reduction potential. Like any phase diagram, the Pourbaix diagram gives the predominant species at the given equilibrium conditions, in this case the pH and Eo.
pH < 3, only +7, +4, +2, and 0 oxidation states available
pH between 4 and 13, +7, +4, +3, +2, and 0 oxidation states
only above pH = 13 can +6 oxidation state be found
Kinetic Considerations
Overpotentials
Even though the thermodynamic potential for a reaction may be favorable, the reaction may proceed slowly. This is because an additional driving force, called the overpotential, is required to make the reaction proceed at a reasonable rate. The overpotential acts like an activation barrier to the kinetic process.
Zn metal does not react rapidly with neutral water, despite having a favorable potential:
Zn2+(aq) + 2 e– → Zn(s) E°= –0.762 V (pH independent)
2 H+(aq) + 2 e– → H2(g) E = –0.0591×pH V
so for the reaction
Zn(s) + 2 H+(aq) → H2(g) + Zn2+(aq) E = –0.0591(7) – (–0.762) = +0.348 V
The overpotential prevents reaction from occurring.
At lower pH, the potential becomes more positive and reaction proceeds:
at pH = 2, E = –0.0591(2) – (–0.762) = +0.644 V
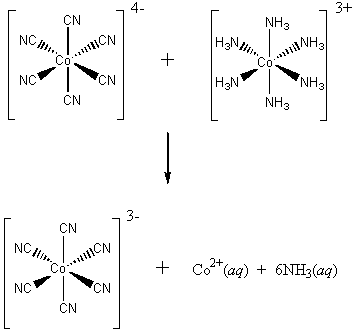
Electron Transfer Mechanisms
Commonly, two types of mechanisms are found: inner sphere and outer sphere
Inner sphere:
The reacting species are linked by a weak covalent bond during the electron transfer.
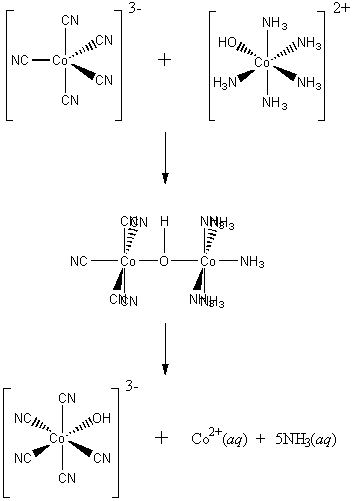
There is room on the Co(II) to accept another species and OH– is capable of bonding to two metals in a bridging situation, thus the Co(II)-OH-Co(III) bridge forms in the transition state.
Outer sphere
Reacting species collide and during the collision the electron transfer takes place
The transition state has no definable bond formation between the two reactants.