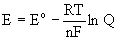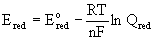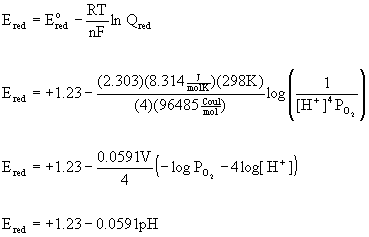Chemistry 401
Oxidation/Reduction Chemistry
Transfer of electrons from oxidized species (loses electrons) to reduced species (gains electrons)
Connections between structure/bonding and reactivity are less obvious than in acid/base chemistry.
Thermodynamic Considerations
The tendency for an electron transfer reaction to occur is embodied in the potential, E, which is directly related to the Gibb's energy, ΔG
ΔG = –nFE
F is Faraday's constant = 96485 Coulombs/mole
n = the number of moles of electrons transferred in the reaction
E is typically more convenient because it can be measured readily using electrochemical techniques.
At standard conditions (1 bar pressure, 25 °C, 1 mole reactant, pH = 0), potentials are denoted E° and are further partitioned (arbitrarily) into reduction potentials for each reacting species:
E° = E°red(reduced species) – E°red(oxidized species)
Reduction potentials are tabulated in a variety of formats.
At nonstandard conditions, the Nernst equation is used to find a reaction potential:
Q is the reaction quotient (mass action expression)
The Nernst equation can also be applied to half-reactions:
Qred is the mass action expression of the reduction half-reaction, ignoring the electrons.
Qred is only a relative value since it is based on the arbitrary partitioning done to obtain E°red
This explains the strong pH dependence of many redox reactions:
O2(g) + 4 H+(aq) + 4 e– → 2 H2O(l)
at pH = 0 (standard conditions) E°red = +1.23 V
at other pH values:
assuming an oxygen pressure of 1 bar