Chemistry 401
Atomic Structure
The key to understanding atomic structure is the so-called "particle/wave duality" of the electron:
Wave properties of electrons are constrained by certain boundary conditions:
1) the electron exists somewhere in space
2) the electron is continuous
3) the electron is finite
When these conditions are imposed upon Schroedinger's equation, the result is quantization
1) each electron can only have certain energies (energy quantization)
2) each electron can only occupy certain volumes of space (spatial quantization)
#2 is more important in most cases for describing bonding
Schroedinger's Equation
Hydrogenic Wavefunctions:
Ψ = two parts : a radial portion and an angular portion (names arise because the mathematical solution is done by transforming to polar coordinates)
Ψ = R(r)ψ(θ, φ)
R(r) = radial function - describes the distance from the nucleus dependence
ψ(θ, φ) = angular function – describes shape and orientation in space
quantization is described by a set of numbers called quantum numbers:
n = principal quantum number, found only in R, distance dependence
l = orbital angular momentum, found in R and ψ, orbital shape
ml = magnetic quantum number, found only in ψ, orientation in space
The quantum numbers are mathematically related
n = 1, 2, 3, 4, ...
l = n-1, n-2, n-3, ..., 0
ml = –l, –l+1, –l+2, ..., l–2, l–1, l
The l quantum number is usually designated by a letter:
lletter designation
0s
1p
2d
3f
4g
Experimental evidence (and relativistic theory) indicates the presence of a fourth quantum number, ms, the spin quantum number
ms = +½ or –½
All the information needed to understand any hydrogenic wavefunction is stored in the set of quantum numbers that describe that wavefunction.
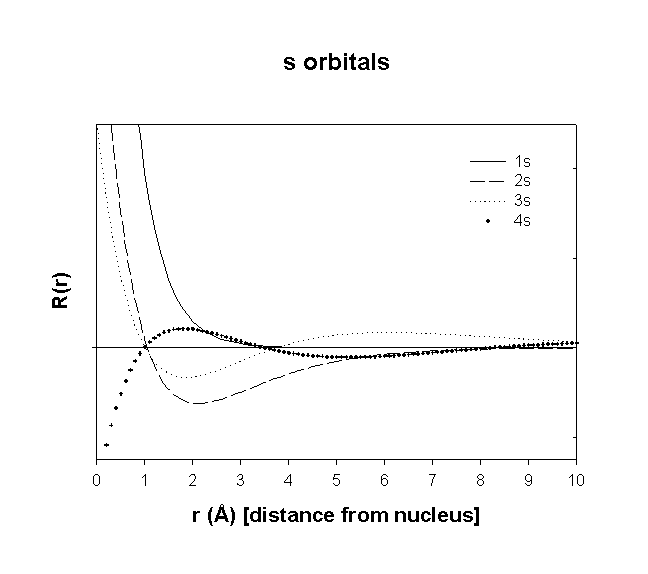
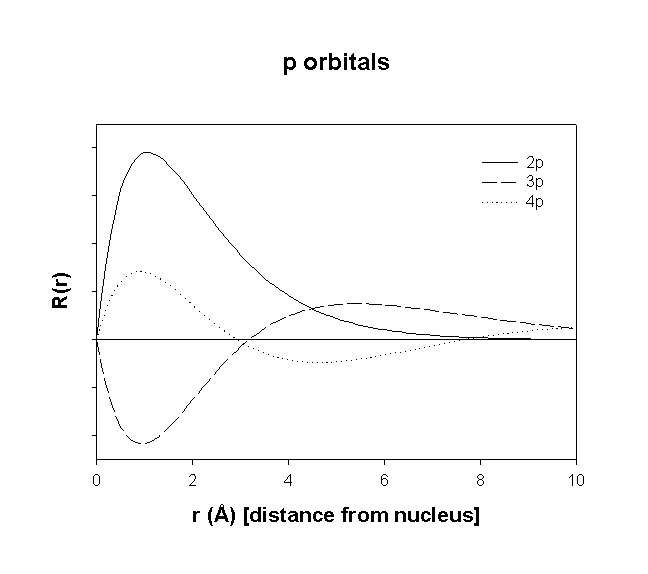
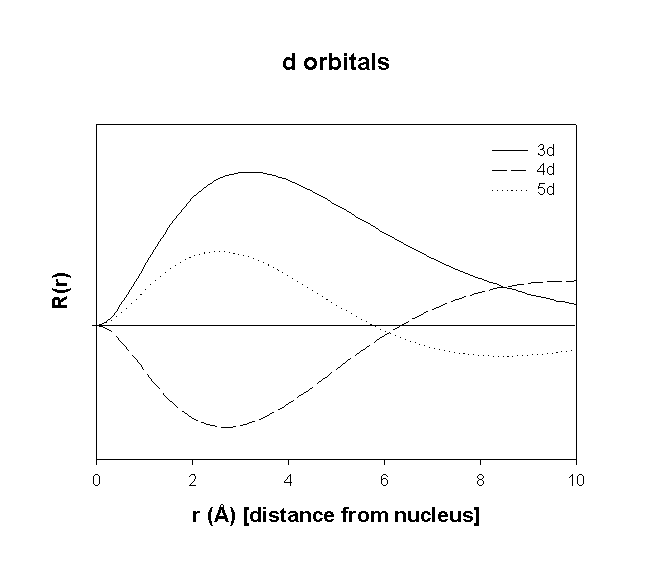
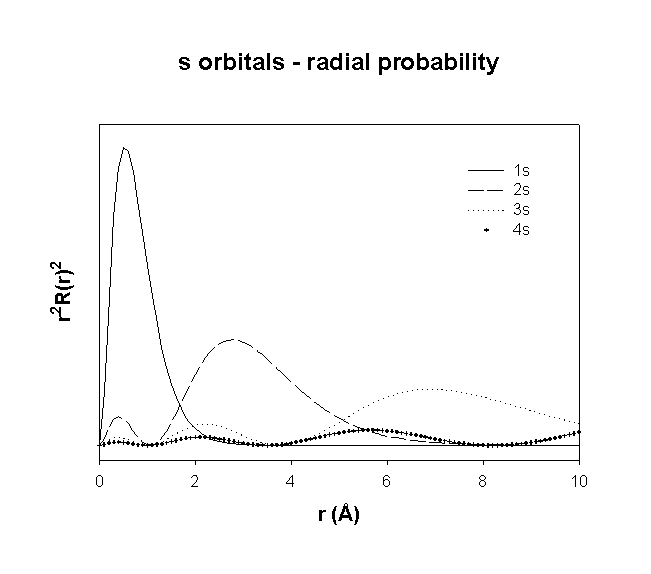
Hydrogenic Radial Wave functions
Angular Wavefunctions : independent of n
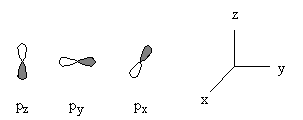
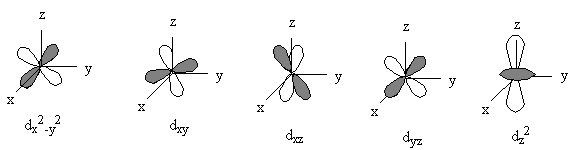
General nodal properties:
total number of nodes = n – 1
total number of planar (or angular) nodes = l
total number of radial nodes = n – l – 1