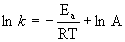
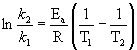
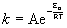
Zero Order Reactions Rate = –k [A] = –kt + [A]o t½ = [A]o/2k k is the rate constant in units of mol·L–1s–1
First Order Reactions Rate = –k[A] ln[A] = –kt + ln[A]o t½ = 0.693/k k is the rate constant in units of s–1
Second Order Reactions Rate = –k[A]2 1/[A] = kt + 1/[A]o t½ = 1/[A]ok k is the rate constant in units of mol–1Ls–1
Rate is the reaction velocity in units of mol·L–1s–1
t is time in units of s
[A]o is the initial concentration of the reactant in units of mol·L–1
t½ is the half-life, the time for the initial concentration to decrease by 50%
[A] is the concentration of reactant at any time t
Arrhenius equation
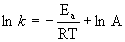
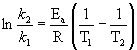
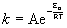
k is the rate constant, in any appropriate units
Ea is the activation energy in units of J/mol or kJ/mol
R is the gas constant, 8.314 J/mol·K
T is the absolute temperature in units of Kelvins, T(K) = T(oC) + 273.15
A is the pre-exponential factor in the same units as the rate constant
Quadratic equation
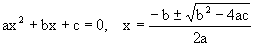
Kp - Kc relationship
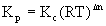
Kp is the equilibrium constant written in terms of partial pressures (atm)
Kc is the equilibrium constant written in terms of concentrations (M)
R is the gas constant, = 0.0821 L·atm/mol·K
T is the absolute temperature in units of Kelvins, T(K) = T(oC) + 273.15
Δn is the change in moles of gas phase materials
Law of Multiple Equilibria: When chemical equations are added, equilbrium constants are multiplied
Reaction 1: A(aq) + B(aq)→← C(aq) + D(aq)Kc1
Reaction 2: C(aq) + E(aq)→← F(aq) + B(aq)Kc2
Net reaction: A(aq) + E(aq)→← D(aq) + F(aq)Kcnet = Kc1×Kc2
definition of pH pH = –log[H3O+]
[H3O+] is the hydronium ion concentration in units of M
definition of pOH pH = –log[OH–]
[OH–] is the hydroxide ion concentration in units of M
definition of pKa pKa = –logKa
Ka is the acid ionization equilibrium constant
definition of % ionization
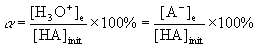
α is the % ionization
[H3O+]e is the equilibrium concentration of hydronium ion in units of M
[A–]e is the equilibrium concentration of the conjugate base anion in units of M
[HA]init is the initial concentration of the weak acid in units of M
Ka - Kb relationship Kw = KaKb for conjugate acid/base pairs
Kw is the equilibrium constant for the autoionization of water
Ka is the acid ionization equilibrium constant
Kb is the base ionization equilibrium constant
Kc for acid-base reactions Kc = KaKb/Kw
Kw is the equilibrium constant for the autoionization of water
Ka is the acid ionization equilibrium constant
Kb is the base ionization equilibrium constant
First Law ΔU = q + w
ΔU is the internal energy in units of J/mol or kJ/mol
q Is the heat transferred in units of J/mol or kJ/mol
w is the work in units of J/mol or kJ/mol
Pressure-volume work w = –PΔV
w is the work in units of L-atm
P is the constant external pressure in units of atm
ΔV is the volume change in units of L
Enthalpy of reaction
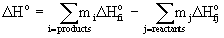
ΔHo is the enthalpy of reaction in units of kJ
ΔHof is the enthalpy of formation in units of kJ/mol
mi is the stoichiometric coefficient for each product
mj is the stoichiometric coefficient for each reactant
Entropy of reaction
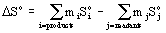
ΔSo is the entropy of reaction in units of J/K
So is the absolute entropy in units of J/mol·K
mi is the stoichiometric coefficient for each product
mj is the stoichiometric coefficient for each reactant
Second Law
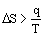 for a spontaneous process
for a spontaneous process
ΔS is the change in entropy in units of J/mol·K
T is the absolute temperature in units of Kelvins, T(K) = T(oC) + 273.15
Third Law S = RlnW
S is the absolute entropy in units of J/mol·K
R is the gas constant, 8.314 J/mol·K
W is the degeneracy of the system (unitless)
Trouton's equation
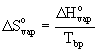
ΔSovap is the entropy change for vaporization in units of J/K
ΔHovap is the enthalpy change for vaporization in units of J
Tbp is the boiling point in units of Kelvins, T(K) = T(oC) + 273.15)
Gibb's Free Energy ΔG = ΔH – TΔS
ΔG is the Gibb's Free Energy change in units of kJ
ΔH is the enthalpy change in units of kJ
ΔS is the entropy change in units of kJ/K
Tbp is the boiling point in units of Kelvins, T(K) = T(oC) + 273.15)
ΔG - w relationship ΔG = wmax
ΔG is the Gibb's Free Energy change in units of kJ
wmax is the maximum work available from a system in units of kJ
ΔG at nonstandard conditions ΔG = ΔGo + RTlnQ
ΔG is the Gibb's Free Energy change in units of kJ
ΔGo is the Gibb's Free Energy change at standard conditions in units of kJ
R is the gas constant, 8.314 J/mol·K
T is the absolute temperature in units of Kelvins, T(K) = T(oC) + 273.15
Q is the reaction quotient expressed with gases in units of atm and concentration in units of M
ΔG - Keq relationship ΔGo =–RTln Keq
ΔGo is the Gibb's Free Energy change at standard conditions in units of kJ
R is the gas constant, 8.314 J/mol·K
T is the absolute temperature in units of Kelvins, T(K) = T(oC) + 273.15
Keq is the thermodynamic equilibrium constant expressed with gases in units of atm and concentration in units of M
van't Hoff equation
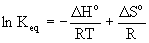
Keq is the thermodynamic equilibrium constant expressed with gases in units of atm and concentration in units of M
R is the gas constant, 8.314 J/mol·K
T is the absolute temperature in units of Kelvins, T(K) = T(oC) + 273.15
ΔHo is the enthalpy change at standard conditions in units of J
ΔSo is the entropy change at standard conditions in units of J/K
Standard cell potential Eocell = Eored + Eoox
Eocell is the standard cell potential in units of V
Eored is the potential for the reduction half-reaction in units of V
Eoox is the potential for the oxidation half-reaction in units of V
Nernst Equation
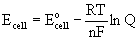
Ecell is the cell potential in units of V
Eocell is the standard cell potential in units of V
R is the gas constant, 8.314 J/mol·K
T is the absolute temperature in units of Kelvins, T(K) = T(oC) + 273.15
n is the number of electrons transferred in the balanced chemical equation
F is Faraday's constant, 96485 C/mol
Q is the reaction quotient expressed with gases in units of atm and concentration in units of M
Eo - Keq relationship
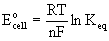
Eocell is the standard cell potential in units of V
R is the gas constant, 8.314 J/mol·K
T is the absolute temperature in units of Kelvins, T(K) = T(oC) + 273.15
n is the number of electrons transferred in the balanced chemical equation
F is Faraday's constant, 96485 C/mol
Keq is the reaction quotient expressed with gases in units of atm and concentration in units of M
Eo - ΔGo relationship ΔGo =–nFEocell
Eocell is the standard cell potential in units of V
ΔGo is the Gibb's Free Energy change at standard conditions in units of J
n is the number of electrons transferred in the balanced chemical equation
F is Faraday's constant, 96485 C/mol
Eo - w relationship wmax = –nFEcell
Eocell is the standard cell potential in units of V
wmax is the maximum work available from a system in units of J
n is the number of electrons transferred in the balanced chemical equation
F is Faraday's constant, 96485 C/mol
Electrolysis nF = At
A is the current passed in units of amps
t is the time in units of s
n is the number of electrons transferred in the balanced chemical equation
F is Faraday's constant, 96485 C/mol