 Final Exam, Spring 2002
Final Exam, Spring 2002 Final Exam, Spring 2002
Final Exam, Spring 20021. Complete and balance the following reactions.
a. HCl(aq) + NaOH(aq)
b. Ba(OH)2(s) + H2CO3(aq)
c. Ti2+(aq) + H2O(l)
d. CH3CH2NH2(aq) + H2O(l)
e. Ag+(aq) + NH3(aq)
f. Pb(NO3)2(aq) + Na2SO4(aq)
g. Hg2(NO3)2(aq) + KCl(aq)
h. C6H5COOH(aq) + H2O(l)
i. ClO3–(aq) + Ag2O(s) → Cl–(aq) + AgO(s) (pH = 14)
j. HNO2(aq) → NO2(g) + NO(g)
2. The following mechanism is proposed for a reaction:
[(P(C6H5)3)3RhCl](s) + H2(g) → [(P(C6H5)3)3RhClH2](s) fast
[(P(C6H5)3)3RhClH2](s) + C2H4(g) → [(P(C6H5)3)3Rh(CH2CH3)ClH](s) slow
[(P(C6H5)3)3Rh(CH2CH3)ClH](s) → [(P(C6H5)3)3RhCl](s) + CH3CH3(g) fast
a. Write the net reaction.
b. The net reaction is an oxidation-reduction reaction. What is oxidized and what is reduced?
c. Predict the rate law.
3. The following reaction was run at 62 °C:
2 N2O5(g) → 4 NO2(g) + O2(g)
The concentration of N2O5 was measured at several different times and the following plots were made:
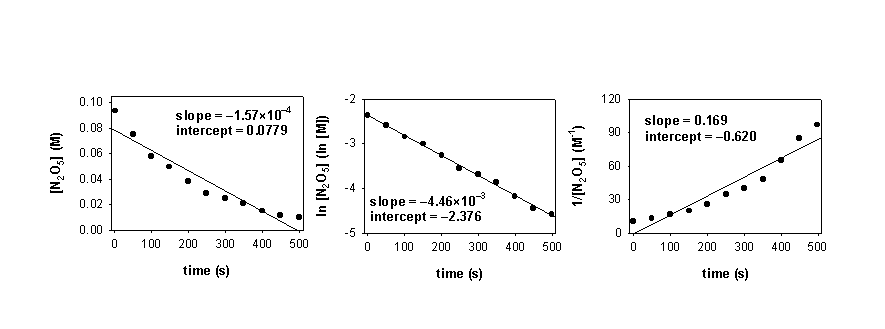
a. What is the order of the reaction? Write the rate law.
b. Find the value of the rate constant and give the units.
4. Estimate the pH of a 0.10 M solution of Ni(NO3)2 at 25 °C.
5. Unlike other solubility reactions, Ksp for sulfides is defined by the reaction:
MS(s) + H2O(l) → ← M2+(aq) + HS–(aq) + OH–(aq)
Potentially useful information:
S(s) + H2O(l) + 2 e– → HS–(aq) + OH–(aq) E° = –0.337
Sn2+(aq) + S(s) + 2 e– → SnS(s) E° = 0.478
Sn2+(aq)ΔH°f = –8.8 kJ/molS° = –17.0 J/mol·K
SnS(s)ΔH°f = –100.0 kJ/molS° = 77.0 J/mol·K
a. Ksp for SnS could be measured using the cell Pt(s) | SnS(s) | Sn2+(aq) | S(s) || S(s) | HS–(aq) | OH–(aq) | Pt(s). Write the oxidation and reduction half-reactions and the net reaction.
b. Find the cell potential for the net reaction for part a. Is the reaction spontaneous or nonspontaneous? Why?
c. Find ΔG° for the reaction in part a.
d. Find Ksp for the reaction in part a.
e. Find ΔH° and ΔS° for the net reaction from part a.
f. Find ΔG° and Ksp using the data from part e. Compare these values to those found in parts c and d.
g. Find molar solubility of SnS.
h. Will the solubility of SnS increase or decrease if the pH is increased. Explain.
i. Find the value for the equilibrium constant at 25 °C for the precipitation reaction given below. (Hint: use the Law of Multiple Equilibria.)
Sn2+(aq) + H2S(aq) + 2 H2O(l) → SnS(s) + 2 H3O+(aq)
j. The reaction shown in part i has a single arrow. Is this justified? Why or why not?
k. Find ΔG° for the reaction in part i.
l. Find ΔH° and ΔS° for the reaction in part i.
m. Using the thermodynamic data from part l to find ΔG°.
n. ΔG° found in parts k and m are quite different. Speculate on a reason that the two values for the Gibb's energy are so different.