 Exam 2B, Spring 2003
Exam 2B, Spring 2003 Exam 2B, Spring 2003
Exam 2B, Spring 20031. Complete and balance the following acid/base reactions.
a. aqueous sulfuric acid plus solid magnesium hydroxide
b. aqueous hydroiodic acid plus water
c. HClO2(aq) + CO32–(aq)
d. HN3(aq) + H2O(l)
a. Mg(OH)2(s) + H2SO4(aq) → 2 H2O(l) + Mg2+(aq) + SO42–(aq)
b. HI(aq) + H2O(l) → H3O+(aq) + I–(aq)
c. HClO2(aq) + CO32–(aq) → ← HCO3–(aq) + ClO2–(aq)
d. HN3(aq) + H2O(l) → ← H3O+(aq) + N3–(aq)
2. Write the chemical reaction needed to determine the pH and give the pH for the following solutions.
a. 0.010 M perchloric acid.
b. 0.0010 M barium hydroxide
a. 0.010 M perchloric acid.
HClO4(aq) + H2O(l) → H3O+(aq) + ClO4–(aq)
[H3O+] = [HClO4] = 0.010 M
pH = –log[H3O+] = –log[0.010] = 2.00
b. 0.0010 M barium hydroxide
Ba(OH)2(aq) → Ba2+(aq) + 2 OH–(aq)
[OH–] = 2×[Ba(OH)2] = 2×[0.0010] = 2.0×10–3 M
pOH = –log[OH–] = –log[2.0×10–3] = 2.70
pH = 14.00 – pOH = 14.00 – 2.70 = 11.30
3. Indicate the stronger acid in each pair. Explain your reasoning in 5 words or less. For the stronger acid, write out the balanced reaction of the acid with water. label the conjugate acid/base pairs for each reaction
a. CCl3COOH vs. CH3COOH
b. H2SO4 vs. HNO3
c. HCrO4– vs. HMnO4
a. CCl3COOH vs. CH3COOH
CCl3COOH because of the Cl electronegativity
CCl3COOH(aq) + H2O(l) → ← H3O+(aq) + CCl3COO–(aq)
b. H2SO4 vs. HNO3
HNO3 because of the N electronegativity
HNO3(aq) + H2O(l) → H3O+(aq) + NO3–(aq)
c. HCrO4– vs. HMnO4
HMnO4 because of charge
HMnO4(aq) + H2O(l) → ← H3O+(aq) + MnO4–(aq)
4. Pyrophosphoric acid is polyprotic, H4P2O7, with Ka1 = 3.0×10–2, Ka2 = 4.4×10–3, Ka3 = 2.5×10–7, and Ka4 = 5.6×10–10. Although it is a weak acid, a 0.010 M solution is greater than 100 % ionized and has a pH of about 1.9. Explain how this can happen, using chemical reactions to supplement your argument.
H4P2O7(aq) + H2O(l) → ← H3O+(aq) + H3P2O7–(aq)
The donation of the first hydrogen ion has a large Ka so the percent ionization is quite high (approximate calculations show ~79%)
H3P2O7–(aq) + H2O(l) → ← H3O+(aq) + H2P2O72–(aq)
The second ionization also has a large Ka - it is not reduced much compared to Ka1 - so the percent ionization for this step is also large (approximate calculations show ~41%)
Thus, the total amount of hydronium ion generated exceeds 0.010 M, explaining the high ionization and the low pH.
5. A 0.10 M solution of hydrogen selenate ion (HSeO4–, Ka = 2.2×10–2) was prepared. Find the pH.
HSeO4–(aq) + H2O(l) → ← H3O+(aq) + SeO42–(aq)
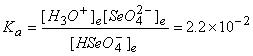
Initial 0.10 0 0
Change –x +x +x
Equilibrium 0.10 – x x x
Approximate? 0.10/2.2×10–2 = 4.4: No
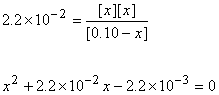
[H3O+]e = 3.7×10–2 M
pH = –log[H3O+] = –log[3.7×10–2] = 1.43